Given the balanced equations representing two chemical reactions:
2 NaBr → 2 Na + Br2
AgNO3 + NaCl → NaNO3 + AgCl
Which types of chemical reactions are represented by these equations?
(1) single replacement and decomposition
(2) single replacement and double replacement
(3) synthesis and decomposition
(4) decomposition and double replacement
(4) decomposition and double replacement
Given the balanced equation representing a reaction:
NH3(g) + O2(g) →N2(g) + H2O(g) + energy
What mass of ammonia completely reacts with 15g of oxygen to produce 32 g of nitrogen and 40g of water?
57g
The unbalanced equation below represents the reaction between silver sulfide and aluminum.
Ag2S + Al → Ag + Al2S3
Determine the gram-formula mass of the aluminum sulfide product.
150 g/mol
The coefficients in a balanced chemical equation represent
(l ) the mass ratios of the substances in the reaction
(2) the mole ratios of the substances in the reaction
(3) the total number of electrons in the reaction
(4) the total number of elements in the reaction
(2) the mole ratios of the substances in the reaction
Which term represents the simplest whole number ratio of atoms of the elements in a compound?
atomic mass
condensed formula
empirical formula
structural formula
C. empirical formula
Given the balanced equation representing a reaction:
2H2 + O2 → 2H2O + energy
Which type of reaction is represented by this equation?
Synthesis
Finish balancing the following equation using the smallest whole-number coefficients:
2Sb2S3(s) + O2(g) → Sb2O3(s) + SO2(g)
2Sb2S3(s) + 9O2(g) → 2Sb2O3(s) + 6SO2(g)
Show a numerical setup for calculating the percent composition by mass of oxygen in Al2O3 (gram-formula mass = 102 g/mol).
(48/102) x 100
What is the mole-to-mole ratio between Al and Al2O3 in the following balanced equation?
8 Al + 3 Fe3O4 ---> 4 Al2O3 + 9 Fe ?
2:1
Which formula is an empirical formula of a compound?
- N2O4
- NH3
- C3H6
- F2
B. NH3
What is the name of the group that contains nonmetals that are most likely to undergo a chemical reaction?
Halogens
What is the missing product in the following equation? Pb(NO3)2 + 2NaI --> PbI2 + 2______
1) Na2O
2) Na2NO3
3) NaNO3
4) Na2(NO3)2
3) NaNO3
Vitamin C, also known as ascorbic acid, has a molecular formula of C6H8O6 and a gram-formula mass of 176 grams per mole.
Determine the number of moles of vitamin C in an orange that contains 0.071 gram of vitamin C.
4.03x10-4 or 0.000403 moles
The balanced equation below represents the reaction between carbon monoxide and oxygen to produce carbon dioxide.
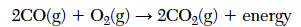
Determine the number of moles of O2(g) needed to completely react with 8.0 moles of CO(g).
4 moles
What is the empirical formula for CH3CH2COOCH3?
C2H4O
The equation below is best classified as which type of chemical reaction?
2C2H2 + 7O2 --> 4CO2 + 6H2O
Combustion
Explain why the balanced equation below represents a conservation of atoms.
6Li + N2 → 2Li3N
Same # of atoms of each element on both sides of the equation
When 5.06 grams of MgSO4·7H2O are heated to at least 300.oC in a crucible by using a laboratory burner, the water molecules are released. The sample was heated repeatedly, until the remaining MgSO4 had a constant mass of 2.47 grams.
Using the lab data, show a numerical setup for calculating the percent composition by mass of water in the hydrated compound.
2.59/5.06 x 100
Given the equation: CH4 + 2O2 --> CO2 + 2H2O
How many moles of oxygen are needed for the complete combustion of 3.0 moles of methane?
6 moles
Determine the molecular formula for a compound that has the empirical formula CH2O and a molar mass of 120. grams per mole.
C4H8O4
For the following reaction, identify the type and state why.
2NaHCO3 + heat --> Na2CO3 + H2O + CO2
Decomposition because one reactant is breaking down into several products
Given the unbalanced equation: Ca(OH)2 + (NH4)2SO4 ---> CaSO4 + NH3 +H2O.
What is the sum of the coefficients when the equation is completely balanced using the smallest whole-number coefficients?
7
Find the % by mass of water in the hydrate NiSO4 · 6H2O
41.1%
Given the reaction: 2C2H2(g) + 5O2(g) --> 4CO2(g) + 2H2O(g), what is the total number of grams of O2(g) needed to react completely with 13 grams of C2H2(g)?
40 grams of O2(g)
What is the empirical formula of a compound that contains 85% Ag and 15% F by mass?
AgF