What is the total number of oxygen atoms in the formula MgSO4 • 7 H2O?
11
What is the gram-formula mass of Ca(OH)2?
74 g/mol
Which formula is an empirical formula?
A) N2O4
B) NH3
C) C3H6
D) P4O10
B) NH3
Which type of chemical reaction is represented by the equation?
F2(g) + 2KCl(aq)-->2KF(aq) + Cl2(g)
Single Replacement
What is the mass of 1.5 moles of CO2?
66 g
Write the formula for sodium sulfate
Na2SO4
What is the gram formula mass of Mg(ClO3)2?
191 g
Given two formulas representing the same compound:
Formula A CH3 Formula B C2H6
Which statement describes these formulas?
A) Formulas A and B are both empirical.
B) Formulas A and B are both molecular.
C) Formula A is empirical, and formula B is molecular.
D) Formula A is molecular, and formula B is empirical.
C) Formula A is empirical, and formula B is molecular.
Which type of reaction does this equation represent?
2NaCl-->2Na + Cl2
decomposition
One mole of O2 has approximately the same mass as one mole of
A) CH4
B) S
C) LiH
D) Cl2
B) S
Write the formula for aluminum iodide
AlI3
What is the gram-formula mass of Fe(NO3)3?
242 g/mol
What is the empirical formula for a compound with the molecular formula C6H12Cl2O2?
C3H6ClO
The coefficients in a balanced chemical equation represent
A) the mass ratios of the substances in the reaction
B) the mole ratios of the substances in the reaction
C) the total number of electrons in the reaction
D) the total number of elements in the reaction
B) the mole ratios of the substances in the reaction
What is the number of moles of CO2 in a 220.-gram sample of CO2 (gram-formula mass = 44 g/mol)?
5.0 mol
What is name for the compound NaClO3
sodium chlorate
Show a numerical setup for calculating the percent composition by mass of oxygen in Al2O3 (gram-formula mass = 102 g/mol).
48/102 x 100
What is the empirical formula of a compound that has a carbon-to-hydrogen ratio of 2 to 6?
A) CH3
B) C2H6
C) C3H
D) C6H2
A) CH3
Which chemical equation is correctly balanced?
A) H2(g) + O2(g) → H2O(g)
B) N2(g) + H2(g) → NH3(g)
C) 2NaCl(s) → Na(s) + Cl2(g)
D) 2KCl(s) → 2K(s) + Cl2(g)
D) 2KCl(s) → 2K(s) + Cl2(g)
What is the number of moles in a 78.8-gram sample of MgCO3 (gram-formula mass = 84.3 g/mol)?
0.935 mol
A correct name for N2O3 is
A) nitrogen (I) oxide
B) nitrogen (II) oxide
C) nitrogen (III) oxide
D) nitrogen (IV) oxide
C) nitrogen (III) oxide
Show a numerical setup for calculating the percent composition by mass of chlorine in PCl3
106/137 x 100
Write the empirical formula of the solid reactant in the equation.
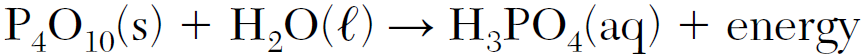
P2O5
Balance the equation:
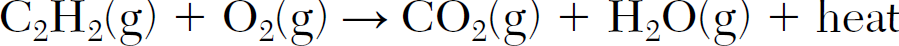

What is the mass in grams of 2.0 moles of NO2?
92