The formula for density.
Density = mass divided by volume
D = m / V
Density is the amount of matter squeezed into a certain amount of space. (True or False)
True
Which of these is a chemical reaction (change)?
A - Tearing paper
B - Burning paper
C - Crumpling paper
B - Burning paper
Which phases of water are found in the water cycle?
A - boiling, evaporation, freezing
B - solid, liquid, gas
C - freezing, melting, evaporation
D - evaporation, condensation, sublimation
B - solid, liquid, gas
(The other incorrect choices are not phases, they are changes in phase.)
The term for a solid turning into a liquid.
The density of water
1 g/cm3 or 1 mL
Ice floats in liquid water. What is the density of ice compared to liquid water?
Ice has a density less than liquid water because liquid water packs more molecules in a certain amount of space compared to ice.
Which of the following is NOT a physical change?
A - Milk turning sour
B - The water cycle
C - Water boiling
A - Milk turning sour is a chemical change.
Which of these is in the liquid phase?
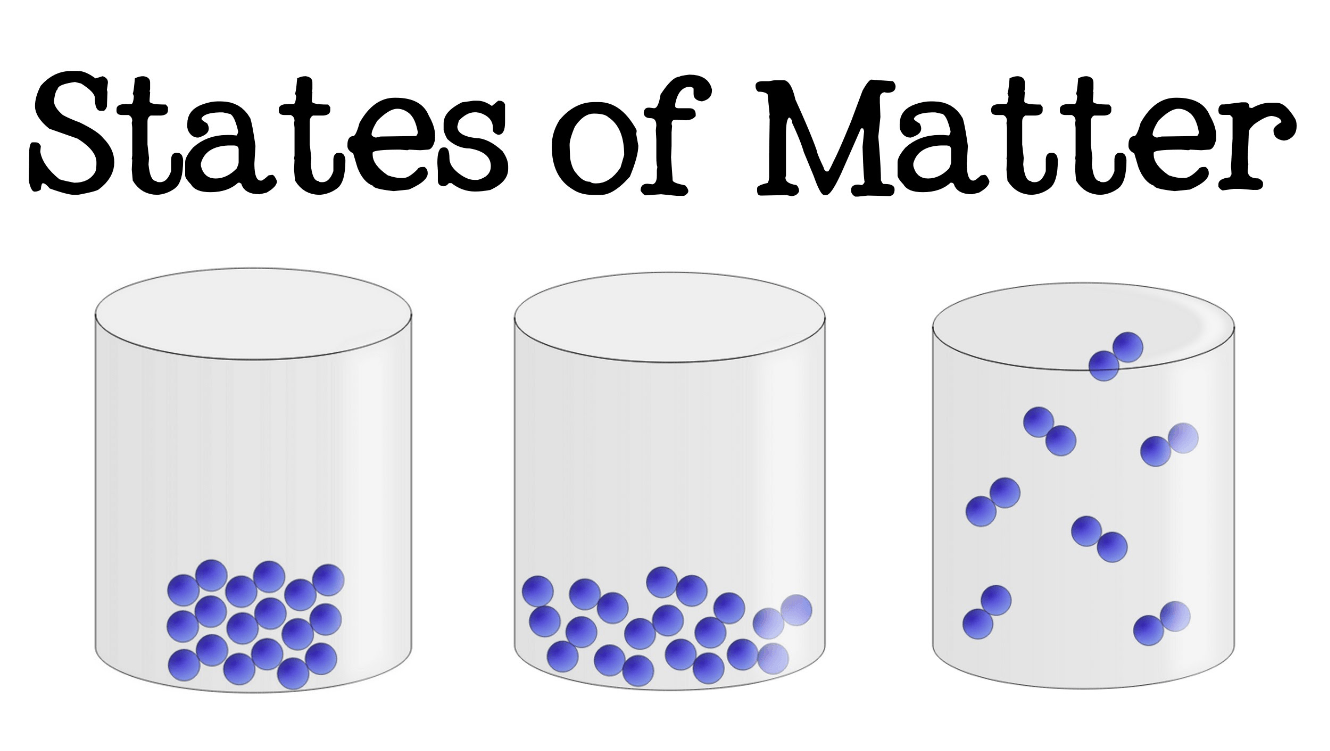
The middle one.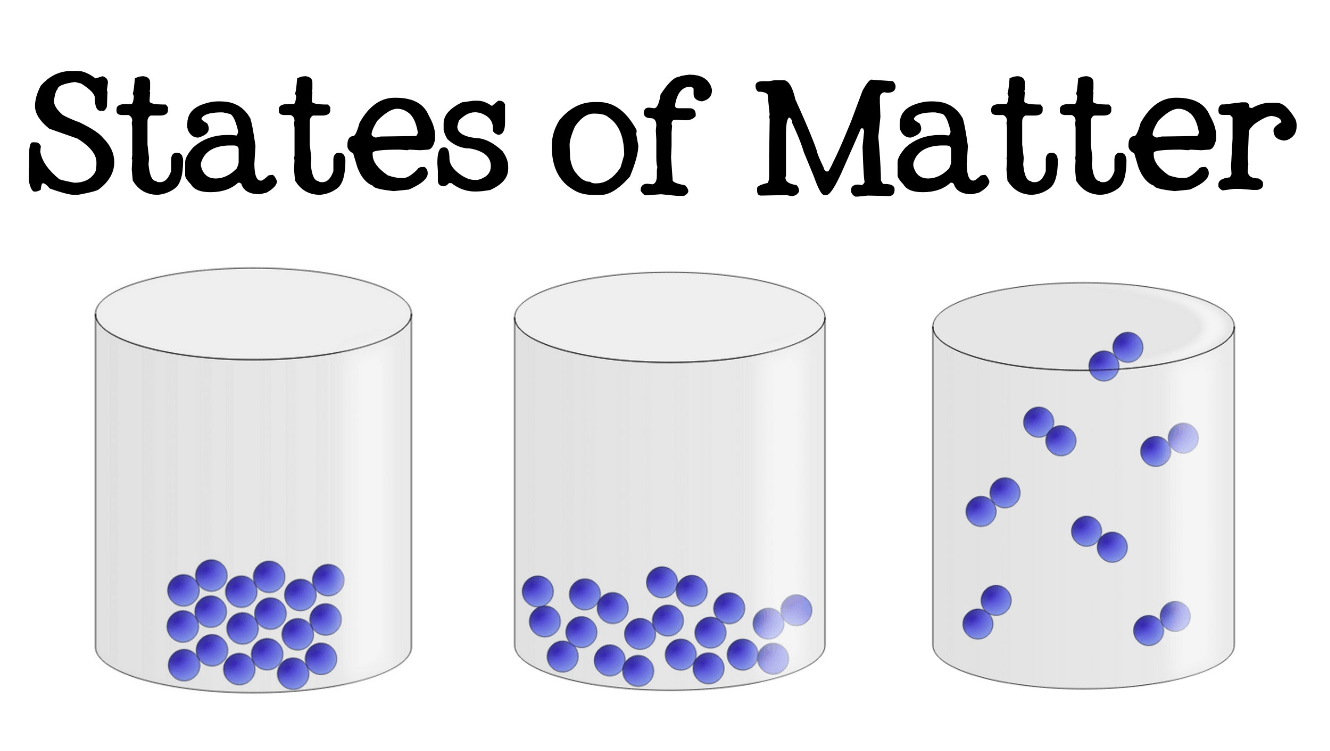
The term for a gas turning into a liquid.
Condensation
The mass of an object is 4 grams. It's volume is 2 cm3. What is the object's density, and how did you calculate it?
Density = mass / Volume
4/2=2 "g/cm"^3
Which of these substance has a density less than 1 g/mL?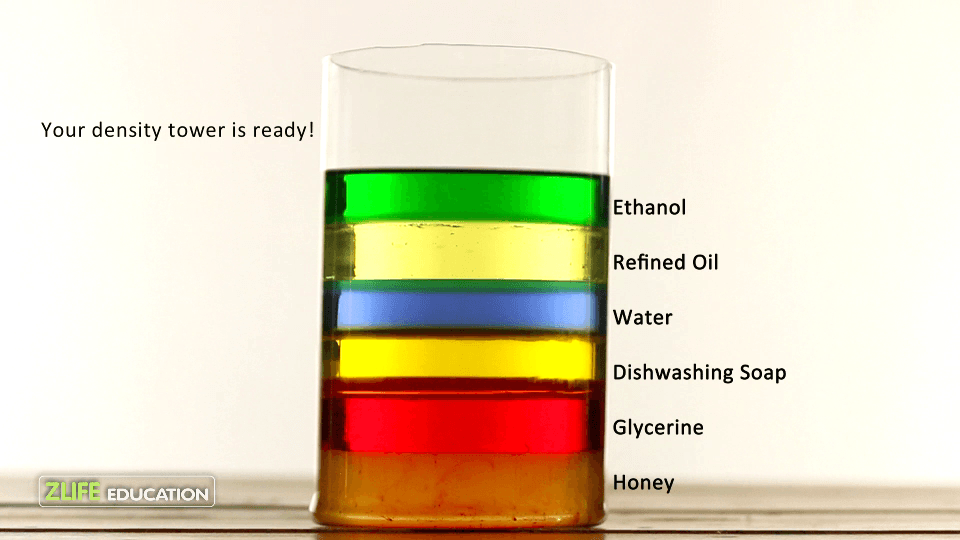
Ethanol and refined oil because they float in water.
State whether each of these is a chemical property or physical property.
A - Paper burns
B - Ice melts at room temperature.
C - Salt dissolves in water
A - Paper burns: chemical property
B - Ice melts at room temperature: physical property
C - Salt dissolves in water: physical property
Which of these have the greatest kinetic energy: the solid, liquid, or gas?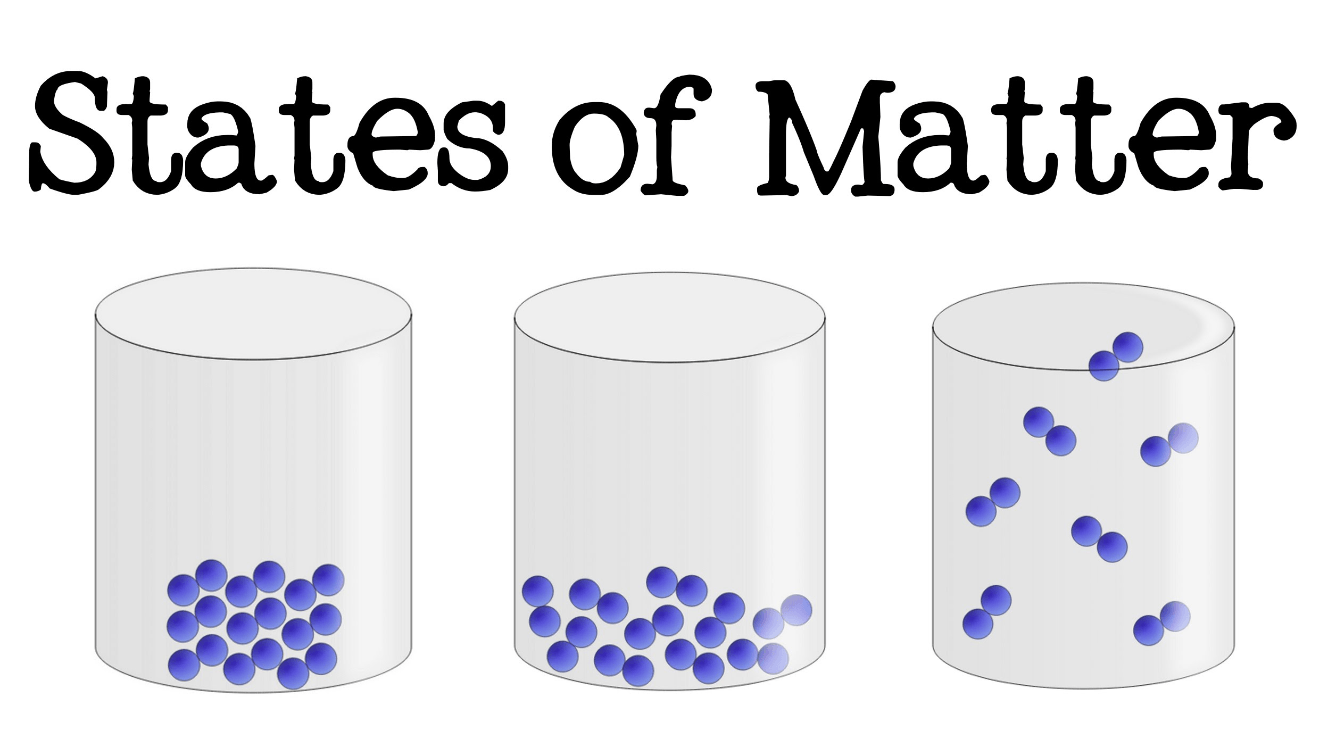
The gas.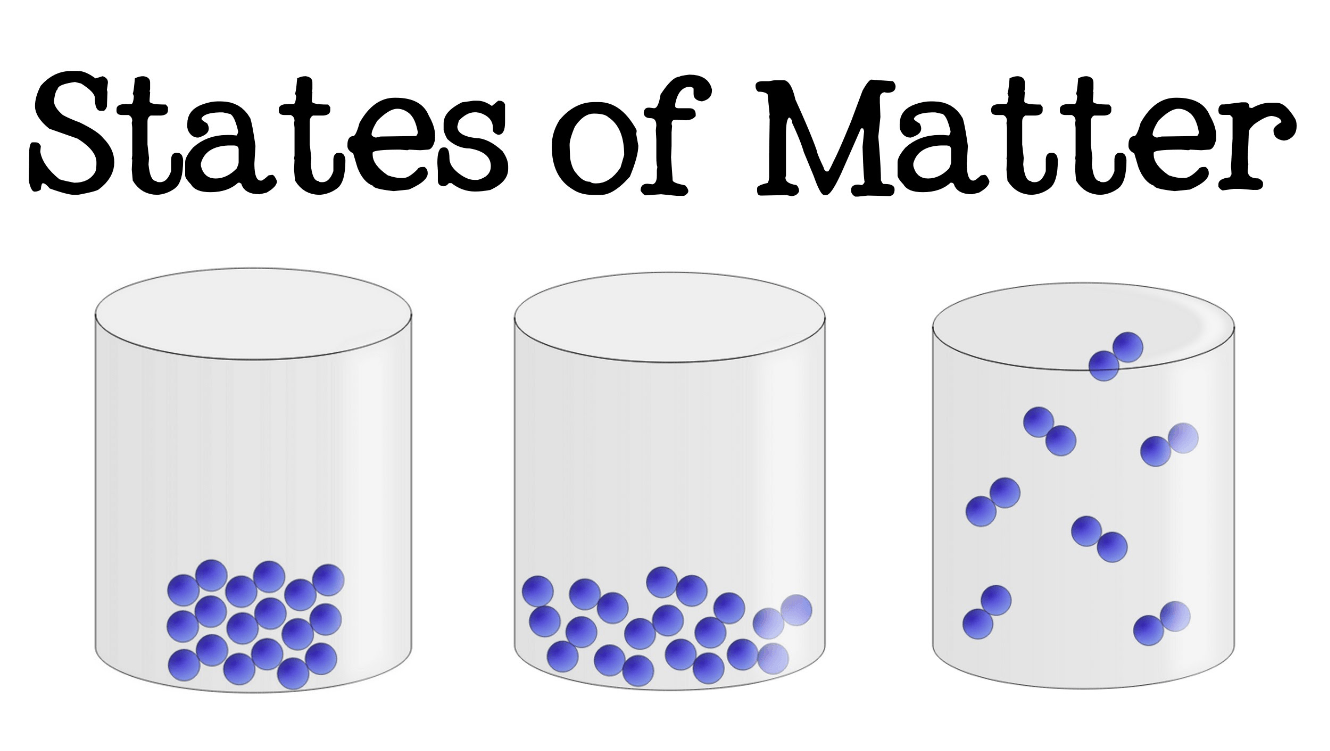
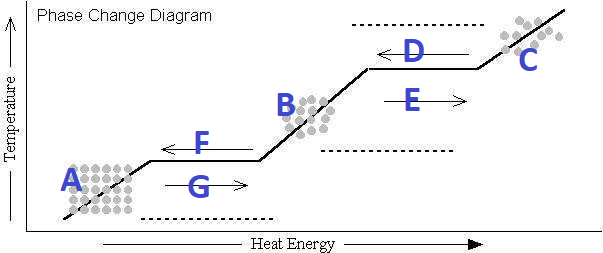
What's happening at G?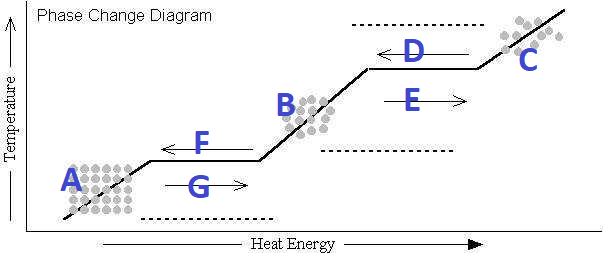
Melting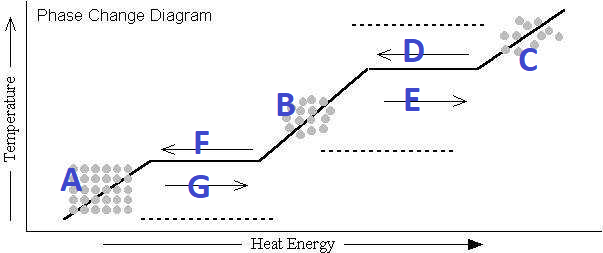
An object has a density of 0.75 g/mL. Will it sink or float in water? How do you know?
The object will float because its density is less than the density of water.
If I drop a small object into these substances, how far will the object sink?
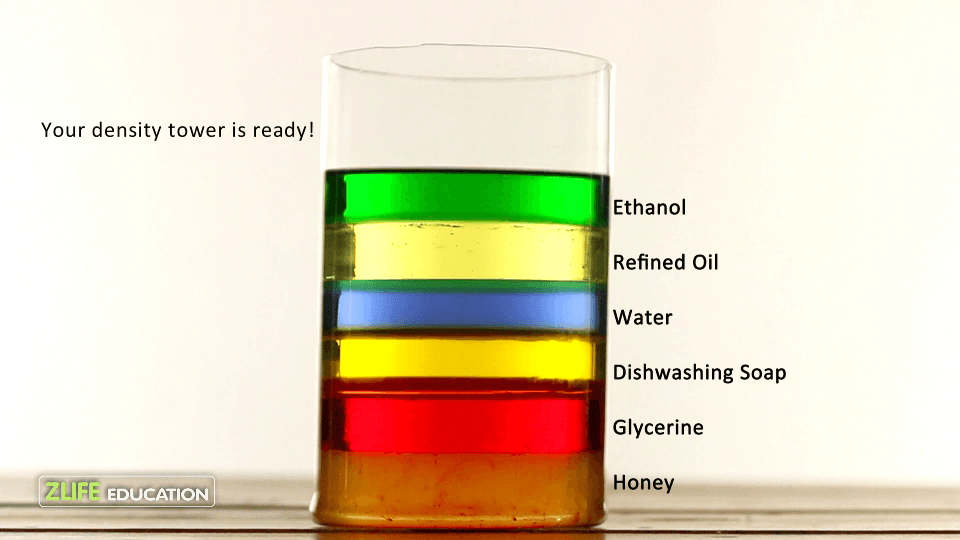
There's no way to tell without knowing the object's density.
Density is a ____ property.
A - physical property
B - chemical property
C - depends on the substance
A - physical property
Why? Because we can observe the density of a substance without changing what it is.
Which state of matter takes up the shape of its container?
Liquid
What's happening at D?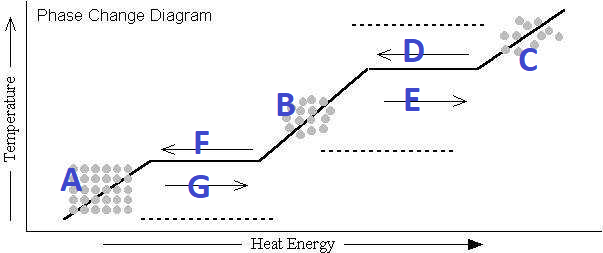
Condensation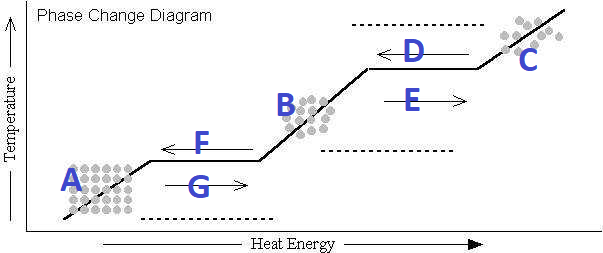
Substance A: mass = 10 g and volume = 5 mL
Substance B: mass = 15 g and volume = 15 mL
Substance C: mass = 1 g and volume = 2 mL
Which substance will float in substance B?
Substance C floats in B because B has a density of 1, and C has a density less than 1
1/2=0.5
Why is water floating above the dish soap, syrup, and honey.
The density of the water is less than the densities of dish soap, syrup, and honey.
Which of the following is a chemical property?
A - Color
B - Density
C - Flammability
D - Solubility
C - Flammability
Other than solids, liquids, and gases, what is the name of the fourth state of matter?
Plasma
What's happening at F?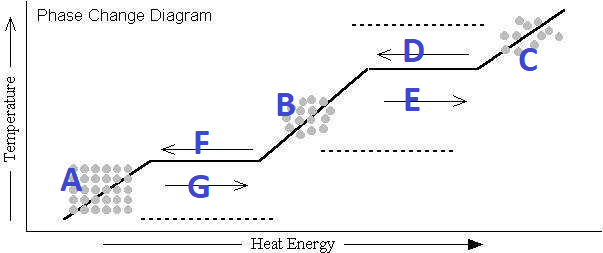
Freezing