What is matter that has a constant chemical composition and characteristics?
Substance
To make lemonade, a student adds sugar to a mixture of lemon juice and water. She notices that initially, all of the sugar dissolves immediately. She then notices that after adding several spoons of sugar, the sugar stops dissolving. She tries stirring the mixture, but the sugar still will not dissolve. Her grandmother fixes the problem by adding more water to the mixture, which causes the sugar to continue dissolving again.
Based on these observations, which of the following most likely increased the amount of sugar particles that dissolved?
Adding water
What is the density of a cube with the following measurements:
Mass: 10 grams
Volume: 5 cm3
2 g/cm3
What is a property that describes how well one substance dissolves in another substance?
Solubility
When nitric acid (HNO3, acid rain) reacts with calcium carbonate in the marble (CaCO3 ), it forms calcium nitrate (CaN2O6).
When sulfuric acid (H2SO4, acid rain) reacts with calcium carbonate in the marble, it forms calcium sulfate (CaSO4).
The marble used to build the Taj Mahal is falling apart. The original designer of the Taj Mahal expected the marble to last through all kinds of weather that was typical for the area 300 years ago. Back then, the air was not polluted and the rain water would not have acids in it that break down marble.
The table below shows the properties of the calcium carbonate and different types of acid rain.
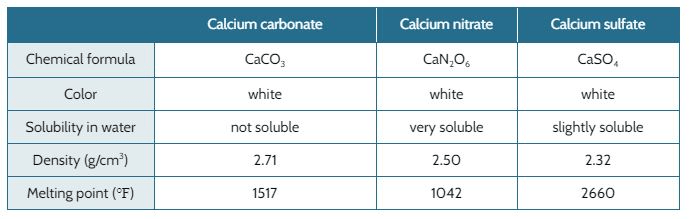
Based on calcium carbonate’s properties, why was it used for building the Taj Mahal?
It's not soluble in water
Which of the following are CHEMICAL properties?
Flammability
Reactivity
Students mix 1 gram of salt into 500 mL of water and place it in a beaker. Then they mix 1 gram of flour into 500 mL of water in another beaker. In the beaker where students mixed the salt, only liquid is visible but in the beaker where students mixed the flour two layers form. The bottom layer is white and the top layer is a clear liquid.
Why were there two separate layers in the beaker with water and flour?
Flour is insoluble in water.
What is on a scale that people can see with their eyes?
Macroscopic
Table salt, also known as sodium chloride (NaCl) would be classified as a:
- Pure substance
- Compound
Compound
When nitric acid (HNO3, acid rain) reacts with calcium carbonate in the marble (CaCO3 ), it forms calcium nitrate (CaN2O6).
When sulfuric acid (H2SO4, acid rain) reacts with calcium carbonate in the marble, it forms calcium sulfate (CaSO4).
The marble used to build the Taj Mahal is falling apart. The original designer of the Taj Mahal expected the marble to last through all kinds of weather that was typical for the area 300 years ago. Back then, the air was not polluted and the rain water would not have acids in it that break down marble.
The table below shows the properties of the calcium carbonate and different types of acid rain.
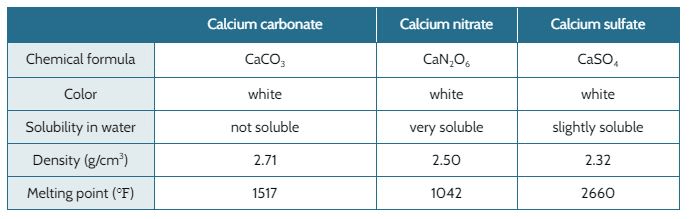
Would calcium carbonate dissolve in water?
Yes, it is soluble in water
While decorating for a family party, a student decides to put floating candles in the backyard fountain. Interested in why the candles can float in the first place, she decides to look at molecular models for candle wax and water.
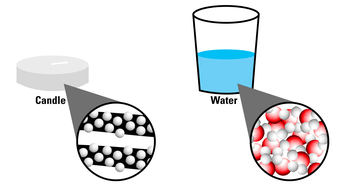
How are the particles in wax spaced compared to water?
They are spaced FURTHER apart
A cup was left on your kitchen table and it's filled with an unknown clear liquid. Which properties would best help you identify what the liquid is?
Color
Smell/ odor
Density
Mass
What is a characteristic of matter called?
Property
Which of the following is a mixture?
- Air
- Gold (Au)
- Oxygen gas (O2)
- Graphite
Air
When nitric acid (HNO3, acid rain) reacts with calcium carbonate in the marble (CaCO3 ), it forms calcium nitrate (CaN2O6).
When sulfuric acid (H2SO4, acid rain) reacts with calcium carbonate in the marble, it forms calcium sulfate (CaSO4).
The marble used to build the Taj Mahal is falling apart. The original designer of the Taj Mahal expected the marble to last through all kinds of weather that was typical for the area 300 years ago. Back then, the air was not polluted and the rain water would not have acids in it that break down marble.
The table below shows the properties of the calcium carbonate and different types of acid rain.
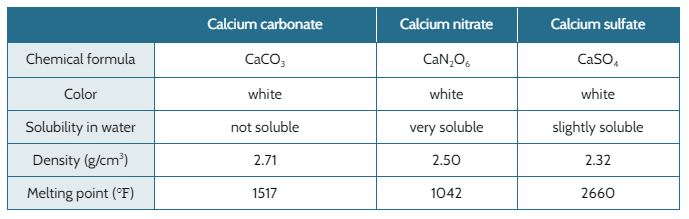
Would calcium sulfate dissolve in water?
Yes, it is slightly soluble in water
While decorating for a family party, a student decides to put floating candles in the backyard fountain. Interested in why the candles can float in the first place, she decides to look at molecular models for candle wax and water.
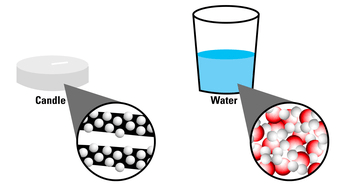
Which substance is MORE dense?
Water
What formula is used to calculate a substance's density?
D = M/V
What is a property that describes the mass of a certain volume of matter?
Density
Both graphite and diamond are made of carbon atoms bonded in different patterns. Are they examples of pure substances or a mixtures?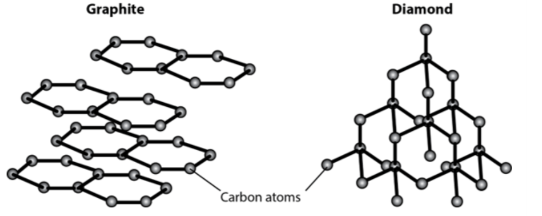
Pure substances (Made of purely of one type of atom/ element)
When nitric acid (HNO3, acid rain) reacts with calcium carbonate in the marble (CaCO3 ), it forms calcium nitrate (CaN2O6).
When sulfuric acid (H2SO4, acid rain) reacts with calcium carbonate in the marble, it forms calcium sulfate (CaSO4).
The marble used to build the Taj Mahal is falling apart. The original designer of the Taj Mahal expected the marble to last through all kinds of weather that was typical for the area 300 years ago. Back then, the air was not polluted and the rain water would not have acids in it that break down marble.
The table below shows the properties of the calcium carbonate and different types of acid rain.
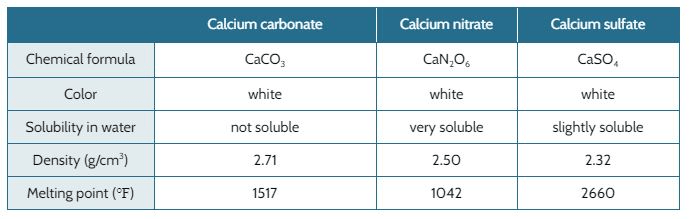
Which type of substance formed from acid rain is more dense?
Calcium nitrate
A pharmacist is preparing aspirin, which is made of acetylsalicylic acid molecules, for a patient who recently suffered a heart attack. However, he mistakenly mixes up the labels on various pill bottles and can no longer tell which bottle contains aspirin. He decides to set up an experiment to determine which bottle contains aspirin.
Which of the following properties should he test for in his experiment?
Any of the following: Density, solubility, melting point, flammability
The table below shows the physical properties of selected metals.
A cube of an unknown metal has a volume of 2.25 cm3 and a mass of 16.2 g. Based on data in the table above, what is the identity of this metal?
Chromium
