Part of the atom involved in a nuclear reaction
Nucleus
Number of electrons shared in this diagram:
8
The number of valence electrons is the same as what part of the periodic table
Group
The subatomic particles located in the nucleus of an atom
Protons and neutrons
What is happening with electrons in a covalent bond?
Electrons are shared
A nuclear reaction results in….
An atom changing into another atom
Type of bonding occurring in this diagram: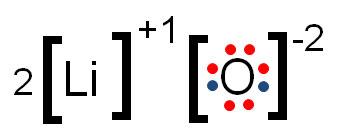
Ionic Bond
Describe or draw trends in electronegativity
Increasing down periods, decreasing down groups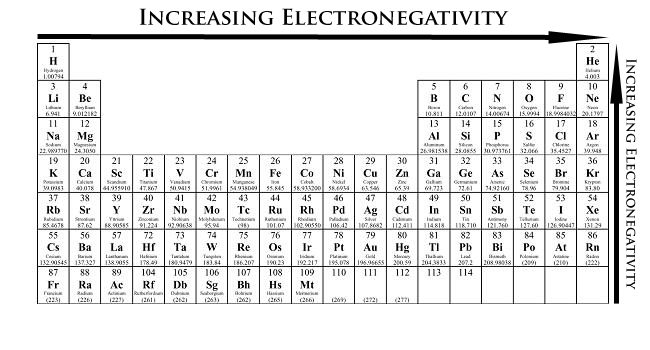
The arrangement of atoms in a periodic table from left to right in a period is based on…
Atomic number
What is happening with electrons in an ionic bond?
Electrons are transferred
Type of Nuclear Reaction this symbol represents: 
Beta Decay
The distribution of charge in this diagram is (even/uneven); (polar/nonpolar): 
Even; nonpolar
How many pairs of electrons are shared between the phosphorus atoms in P2
3 pairs
Atomic mass of an atom that has 8 protons, 8 neutrons, and 8 electrons
16 u
Which of the following has both ionic and covalent bonds: BaCO3, NaCl, H2O, KI
BaCO3
What is happening with energy and mass in a fission reaction?
Mass is converted into energy
Draw a lewis dot structure for SH2
Magnesium and Calcium are chemically similar due to what reason (think electrons)
2 valence electrons
The atom is period 5 that is unreactive at STP is…
Xenon
Which of the following molecules is polar covalent: CO2, C2, NH3, CH4
CO2