What subatomic particle, which has a negative charge, orbits the nucleus of an atom?
Electron
Which of these represents a strong, negative correlation?
a. r = 0.3
b. r = -0.45
c. r = -0.95
r = -0.95
Slope
An element/ atom is determined by the number of which part of the atom?
Protons
Is this survey question qualitative, or quantitative?:
"What is your shoe size?"
Quantitative
If a correlation is weak, are the points spread out or close together?
Spread out
8
Two variables have a strong positive correlation. (Example: average income in a neighborhood and number of local parks). Does this mean that one of these things causes the other?
NO! Correlation does NOT equal causation.
An atom of Fluorine (F) has 9 protons and 10 neutrons. What is its mass number?
19
An Atom has 16 protons, 23 Neutrons and 34 electrons. As an Ion, what is the charge?
-18
What is an appropriate correlation coefficient (r-value) for the following scatter plot?:
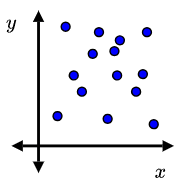
Any number between -0.20 and +0.20
When a neutral atom of Oxygen (O) has 8 protons and 8 electrons, suddenly gains two electrons to become an ion, what is the charge?
-2
Name the type of chemical bond that involves the sharing of valence electrons between atoms.
Covalent bonds
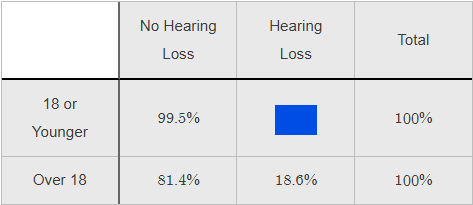
What percentage should appear in the blue box?
0.5%
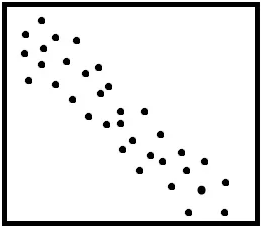
What kind of association is this?
Negative
How many valence electrons (dots) does the element Antimony have?
5
When filling out a two-way frequency table, what should appear in the bottom-right box?
Total, Grand Total, or 100%
Every element in column 13 of the periodic table has how many valence electrons?
3
Draw the bond between Calcium and Iodine

17%
A correlation coefficient r is equal to -0.43. Classify this as either weak or strong, and as positive or negative.
Weak & Negative
Draw the bond for the following:
H2O

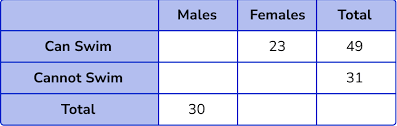
How many total females were surveyed?
50
The Isotope Gold- 300 (this is clearly fake), has how many neutrons?
221 Neutrons