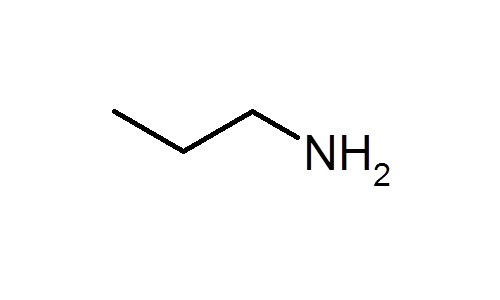
What is a primary amine?
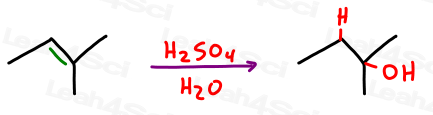
What is hydration?
Losing electrons.
What is oxidation?
CaSO4 -> CaS + O2
What is CaSO4 -> CaS + 2O2 ?
NaCl
What is +1 for Na, and -1 for Cl?

What is a carboxylic acid?
:max_bytes(150000):strip_icc()/methanecombustion-58e3e6005f9b58ef7e0daa10.jpg)
What is a combustion reaction?
Gaining electrons.
What is Reduction?
K2O + H2O -> KOH
What is K2O + H2O -> 2 KOH ?
Fe2O3
What is +3 for Fe, and -2 for O?
C3H8
What is an alkane?

What is a dehydration reaction?
2As(s) + 3Cl2 (g) ![]() 2AsCl3
2AsCl3
(In regards to the As)
What is oxidation?
PCl5 + H2O -> POCl3 + HCl
What is PCl5 + H2O -> POCl3 + 2 HCl ?
SO2
What is +4 for S, and -2 for O?

What is an ether?

What is a decomposition reaction?
3 Hg2+ + 2 Fe (s) ![]() 3 Hg2 + 2 Fe3+
3 Hg2 + 2 Fe3+
(In regards to the mercury)
What is reduction?
Mg + N2 -> Mg3N2
What is 3 Mg + N2 -> Mg3N2 ?
Ni(OH)2
What is +2 for Ni, -2 for O, and +1 for H?

What is an aromatic carboxylic acid?
2 O2 + Sb + 2 H2O ![]() 2 H2O2 + SbO2-
2 H2O2 + SbO2-
What is an oxidation/reduction reaction?
2 H+ + H2O2 + 2 Fe2+ ![]() 2 Fe3+ + 2 H2O
2 Fe3+ + 2 H2O
(In regard to the O)
What is reduction?
Fe2O3 + CO ![]() Fe + CO2
Fe + CO2
What is Fe2O3 + 3 CO ![]() 2 Fe + 3 CO2 ?
2 Fe + 3 CO2 ?
Cr2O7-2
What is +6 for Cr, and -2 for O?