What subatomic particle identifies an element? hint: this # of particles NEVER changes
Protons
Name the 3 subatomic particles, their charges, and where each is located in an atom
Neutron Neutral Charge-nucleus
Proton Positive Charge- Nucleus
Electron Negative Charge-outside of the nucleus
Which 2 types of elements are Ionic Bonds formed between?
1 Metal and 1 Non-metal
Or
1 positively charged element AND 1 negatively charged element(or polyatomic ion)
Name the model that illustrates an element by using the atomic symbol surrounded by dots to represent valence electrons. 
Electron Dot Diagram OR Lewis Dot Structure
What is the name of the Ionic Compound NaCl ?
Sodium Chloride
Name any group of periodic elements. Not the #'s but the names of the groups.
Alaki Earth Metals, Alkaline Earths, Noble Gasses, Transition Metals, Metalloids, Halogens, etc.
Which subatomic particle has the MOST mass of all 3?
Neutron
What is the charge of the element Neon or Ne and why?
0 or Neutral- because it has a full outer shell/electron orbital/energy level.
Which type of compounds are double and triple bonds most commonly found in?
covalent compounds
Which Law represents the reason why we must balance chemical equations?
The law of conservation of mass
What is matter?
What is anything that has mass and takes up space
Which 2 particles make up an elements mass #? Where are they Located?
Protons and Neutrons. In the nucleus
What 2 types of elements make up a covalent compound?
What is the name of the following model of an atom?
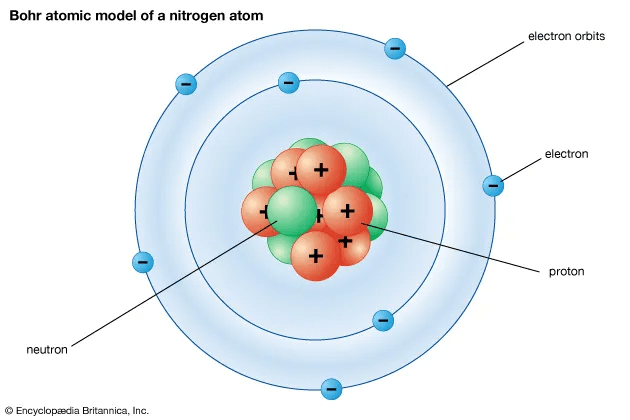
The bohr model
List the steps for naming an Ionic compound?
1. Name the first element/polyatomic ion as is
2. Add the suffix "-ide" to the end of the 2nd element*
*if it's a polyatomic ion and not a single element name the polyatomic ion as it's listed on the back of the periodic table.
What are 4 of the 5 states of matter?
Solid, Liquid, Gas, Plasma, Bose-Einstein Condensates
What is the difference between an ion and an isotope?
In an ion the atom has gained or lost electrons so the number of electrons is no longer equal to the number of protons. Isotopes have the same number of protons and electrons but a different number of neutrons, resulting in a different mass number.
Determine the ionic compound for Mg and Cl
MgCl2
How are ionic compounds named?
Name the first element as listed on the PT
Add the suffix "-ide" to the end of any single element
If any polyatomic ions are within the compound name them as listed on the back of your periodic table.
Balance the following Equation


What is the term for an element with a variety of "types" which differs in the # of neutrons it has. Name the term and give me any example.
Isotope. Carbon-11
Which electron orbital is the ONLY one that cannot have 8 electrons within it?
The very 1st electron orbital
How do you identify the most common isotope?
The most common isotope is the one represented on the periodic table. ie. Carbon's most common isotope is Carbon-11
What charge does Pb(III) have?
3+
What is the name of the following Ionic Compound
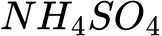
Ammonium Sulfate