A chemist determined by measurements that 0.030 moles of calcium participated in a chemical reaction. Calculate the mass of calcium that participated in the chemical reaction.
1.2 g
What is the total number of hydrogen atoms on the right-hand side of this chemical equation?
8Al + 3NaNO3 + 5NaOH + 18H2O → 8NaAl(OH)4 + 3NH3
41
Classify each chemical compound as ionic or molecular:
CuCl, BI3, Pb(MnO4)2
ionic, molecular, ionic
The following chemical reaction takes place in aqueous solution:
FeCl3(aq) + 3KOH(aq) → FeOH3(s) + 3KCl(aq)
What are the spectator ions?
K+ and Cl-
A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation.
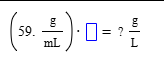
(1*10^3 mL)/(1 L)
A chemist measured the amount of Ca3(PO4)2 produced during an experiment. She finds that 406. g is produced. Calculate the number of moles of product.
1.31 moles
Balance the chemical equation below using the smallest possible whole number stoichiometric coefficients. What is the coefficient for water?
Si4H10 + O2 → SiO2 + H2O
10
4 Si4H10 + 13 O2 → 8 SiO2 + 10 H2O
A chemist prepares a solution of zinc oxalate (ZnC2O4) by measuring out 0.00291μmol of zinc oxalate into a 200.mL volumetric flask and filling the flask to the mark with water. What is the concentration?
1.46E-7 M
(0.00291*10^-6mol)/(200*10^-3L)
When Na2S (aq) and Zn(NO3)2 (aq) is mixed, a precipitate forms. What is the precipitate's formula?
ZnS
Assign charges (positive, negative, or neutral) to the three particles (A, B, & C) based upon the following pictures:

neutral, positive, positive OR
neutral, negative, negative
Measurements of sample of an unknown compound (MgxCly) show that it contains 9.90mol of magnesium and 19.74mol of chlorine. What is the chemical formula?
MgCl2
Using the balance equation, calculate the moles of water produced by the reaction of 0.075mol of octane (C8H18).
2C8H18 + 25O2 --> 16CO2 + 18H2O
0.68 mol
A chemist must dilute 79.0mL of 3.03M aqueous zinc nitrate (ZnNO32) solution to 2.00M. Calculate this final volume, in liters.
0.120L
V_f=(0.0790L xx 3.03M)/(2.00M)
Predict the products of the reaction below. Be sure your equation is balanced.
HNO3 + Ca(OH)2 → ?
2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O
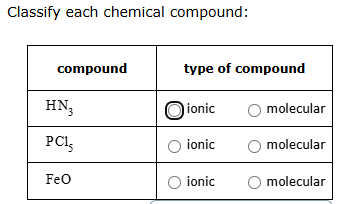
molecular, molecular, ionic
Calculate the number of hydrogen atoms in a 80.0g sample of hydrazine (N2H4).
6.01E24 H atoms
Using the following balanced equation, determine the mass of water (18.02 g/mol) consumed by the reaction of 1.21g of carbon dioxide (44.01 g/mol)?
6CO2 + 6H2O --> C6H12O6 + 6O2
0.495 g H2O
1.21 g CO_2 xx (molCO_2)/(44.01 g) xx(6molH_2O)/(6molCO_2) xx(18.01g)/(mol H_2O)
A chemistry student weighs out 0.0559g of HClO (52.45 g/mol) into a 250.mL volumetric flask and dilutes to the mark with distilled water. He titrates the acid with 0.1000M NaOH solution. Calculate the volume of NaOH (in mL) needed to reach the equivalence point.
HClO + NaOH --> NaClO + H2O
0.0106L = 10.6 mL
0.0559gHClO xx (mol HClO)/(52.45g)xx(1molNaOH)/(1molHClO)xx(1L)/(0.1000molNaOH)
Some chemical reactants are listed in the table below. Complete the table by filling in the oxidation state of the highlighted atom.
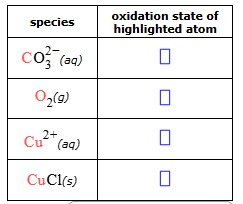
+4, 0, +2, +1
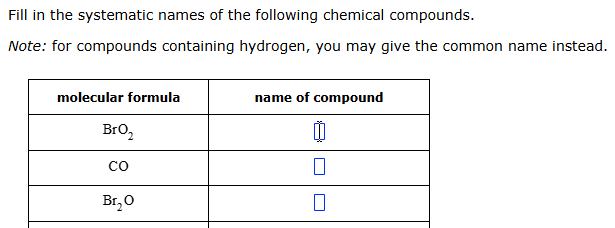
bromium dioxide, carbon monoxide, dibromium oxide (or monoxide)
If the chemical formula for iron(II) hydroxide is Fe(OH)2, calculate the mass percent of oxygen in iron(II) hydroxide.
35.6% Oxygen
Suppose you have 1.0 mol of NO2 and 11.0 mol of H2O in a reactor. How many moles of HNO3 can be produced?
3NO2 + H2O --> 2HNO3 + NO
0.66 mol
11molH_2O xx (2molHNO_3)/(1molH_2O)
1.0 mol NO_2 xx (2 mol HNO_3)/(3molNO_2)
Calculate the concentration (mg/L) of iron(III) chloride (162.19 g/mol) contaminant in the original groundwater sample, if a 250.mL sample produces 5.9mg of silver chloride (143.32 g/mol) when treated with silver nitrate.
FeCl3 + 3AgNO3 → 3AgCl + Fe(NO3)3
2.22E-3g/L = 2.22mg/L
(5.9*10^-3gAgCl)/(0.250L)xx(molAgCl)/(143.32gAgCl)xx(1molFeCl_3)/(3molAgCl)xx(162gFeCl_3)/(molFeCl_3)
Classify each chemical reaction as combination, decomposition, or single or double displacement:
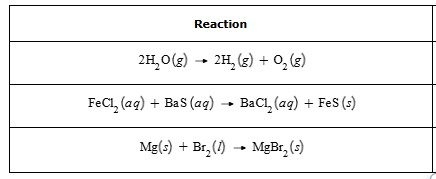
decomposition, double replacement (precipitation), combination (synthesis)
A student sets up the following equation to solve a problem in solution stoichiometry. (The ? stands for a number the student is going to calculate.) What are the units of the student's answer?
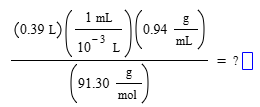
mol