The typical pressure of carbon dioxide in an unopened soda can is 3.45 atm. Calculate the typical pressure in kPa. 1 atm = 101,325 Pa
350. kPa
A reaction at 14.0°C evolves 571mmol of CO2 gas. Calculate the volume (L) of CO2 gas that is collected. You can assume the pressure in the room is exactly 1 atm.
13.5 L
A a slice of cooked bacon contains 3.60 x 104 calories. How many kcal and kJ does the slice contain?
36.0 kcal and 151 kJ
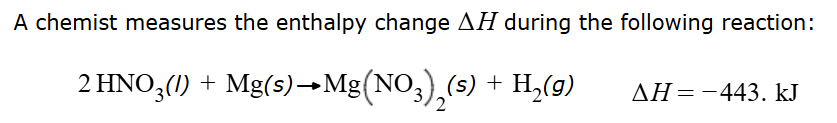
What is the following enthalpy change?
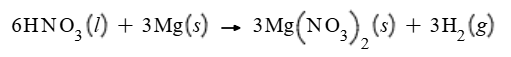
-1.33E3 kJ
What is the total number of hydrogen atoms on the right-hand side of this chemical equation?
8Al + 3NaNO3 + 5NaOH + 18H2O → 8NaAl(OH)4 + 3NH3
41
A cylinder is filled with 10.0L of gas and a piston is put into it. The initial pressure of the gas is measured to be 181kPa. The piston is now pushed down, compressing the gas, until the gas has a final volume of 5.90L. Calculate the final pressure of the gas in kPa.
307 kPa
Some N2 gas (blue molecules) is mixed with some O2 gas (red molecules). The total pressure of the mixture is measured, and found to be 1500.torr What is the mole fraction and partial pressure of N2 (torr)?
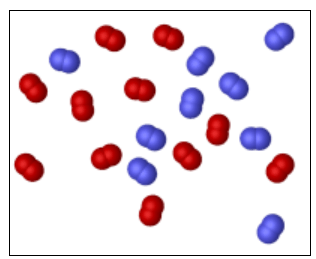
.
mole fraction = 0.4500; partial pressure = 675.0 torr
A mixture of krypton and hydrogen gas is expanded from a volume of 12.0L to a volume of 89.0L, while the pressure is held constant at 85.0atm. Calculate the work done on the gas mixture. Be sure your answer has the correct sign (positive or negative).
Note: 101.3J = 1 atm*L
-663 kJ
w=-pDeltaV=-(89.0L-12.0L) xx 89.0atm=6,545L*atm
w=-6,545atm*Lxx(101.3J)/(atm*L)xx(1kJ)/(1000J)
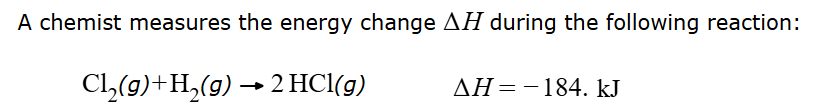
Is the reaction endothermic or exothermic? Is heat absorbed or released by the system? You must answer both questions correctly for points.
exothermic and heat is released by the system.
When Na2S (aq) and Zn(NO3)2 (aq) is mixed, a precipitate forms. What is the precipitate's formula?
ZnS
An arctic weather balloon is filled with 19.9L of helium gas inside a prep shed. The temperature inside the shed is 6.°C. The balloon is then taken outside, where the temperature is −39.°C. Calculate the new volume of the balloon in liters.
16.7L
Calculate to three significant digits the density (g/L) of ClF5 gas at exactly 15°C and exactly 1atm. You can assume chlorine pentafluoride gas behaves as an ideal gas under these conditions.
5.52 g/L
A chemist carefully measures the amount of heat needed to raise the temperature of a 835.0g sample of a pure substance from 23.7°C to 39.0°C. The experiment shows that 60.0kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance as J/(g*K)?
4.70 J/(g*K)
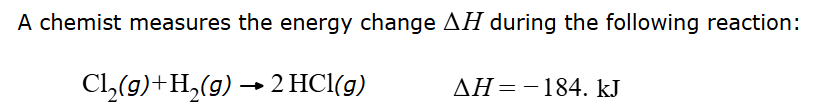
Suppose 13.0 grams of Cl2 is reacted. Calculate how much heat will be transferred.
30.1 kJ
Measurements of sample of an unknown compound (Mg2Cly) show that it contains 9.90mol of magnesium and 19.74mol of chlorine. What is the chemical formula?
M2Cl4
Suppose the temperature of a sample of methane gas is raised from −11.0°C to 22.0°C, and at the same time the pressure is changed. If the initial pressure was 1.7atm and the volume increased by 50.0%, what is the final pressure in atm?
1.3 atm
Here is a graph of the probability of an atom moving with a particular speed, for a sample of krypton gas at −5.°C. What is the most likely speed (m/s) of a Kr atom in this sample?
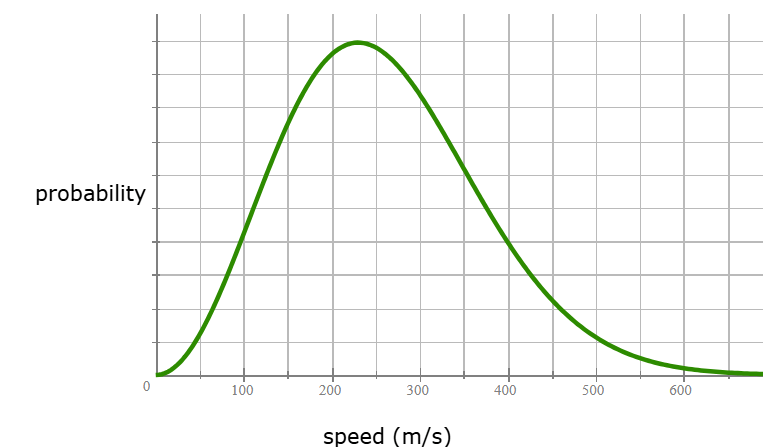
231 m/s +/- 25 m/s
Calculate the energy required to heat 488.0g of graphite from 11.2°C to 21.6°C. Assume the specific heat capacity of graphite under these conditions is 0.710J·g−1·K−1.
3.60kJ
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. In the first step, nitrogen and hydrogen react to form ammonia:
N2(g) + 3H2(g) → 2NH3(g), ΔH = −92.kJ
In the second step, ammonia and oxygen react to form nitric acid and water:
NH3(g) + 2O2(g) → HNO3(g) +H2O(g), ΔH = −330.kJ
Calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions.
-376 kJ
A chemist must dilute 79.0mL of 3.03M aqueous zinc nitrate (ZnNO32) solution to 2.00M. Calculate this final volume, in liters.
0.120L
V_f=(0.0790L xx 3.03M)/(2.00M)
Hydrogen gas and nitrogen gas react to form ammonia gas. What volume (mL) of ammonia would be produced by this reaction if 2.81mL of nitrogen were consumed?
N2(g) + 3H2(g) --> 2NH3(g)
5.62 mL
A 7.00L tank at 25.9°C is filled with 12.4g of N2F2 gas and 9.77g of SF6 gas. You can assume both gases behave as ideal gases under these conditions. Calculate the the total pressure (atm) of the gases as well as the mole fraction and partial pressure (atm) of the N2F2 gas.
Pt = 0.893 atm; XN2F2 = 0.737; PN2F2 = 0.659 atm
A 56.4g sample of aluminum is put into a calorimeter (see sketch at right) that contains 150.0g of water. The aluminum sample starts off at 93.2°C and the temperature of the water starts off at 15.0°C. When the temperature of the water stops changing it's 20.5°C. The pressure remains constant at 1atm. Calculate the specific heat capacity of aluminum according to this experiment (J/(g*°C).
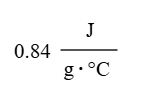
Calculate the reaction enthalpy of this reaction under standard conditions:
2CH3OH(l) + 3O2(g) → 2CO2(g) + 4H2O(l)
Round your answer to the nearest kJ.
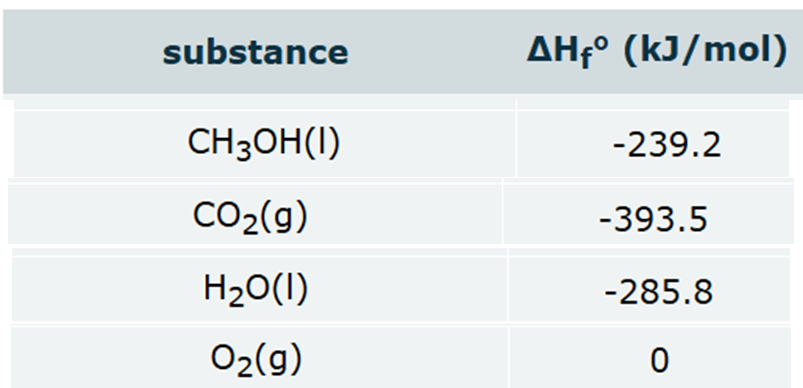
-1452 kJ
Using the following balanced equation, determine the mass of water (18.02 g/mol) consumed by the reaction of 1.21g of carbon dioxide (44.01 g/mol)?
6CO2 + 6H2O --> C6H12O6 + 6O2
0.495 g H2O
1.21 g CO_2 xx (molCO_2)/(44.01 g) xx(6molH_2O)/(6molCO_2) xx(18.01g)/(mol H_2O)