Are all atoms isotopes?
Yes
How many moles is 35.4 g of Li2CO3?
0.479 mol
Balance the following reaction
2NaOH + H2SO4 -> Na2SO4 + 2H2O
0.5552 km^3 to cm^3
5.552*10^14 cm^3
What is true about the structure of an atom?
Atoms are the smallest indivisible particles of matter
Most of an atom is empty space besides the nucleus
Electrons are located in the nucleus
Atoms are mostly solid
All atoms of an element have the same exact structure
2
What are two things wrong with an isotopic notation of hydrogen-2 that has a mass number of 1 and an atomic number of 2 ?
It should have a mass number of 2 and an atomic number of 1
What is a hydrocarbon?
A molecule containing only carbon and hydrogen
Nitric acid (HNO3) can be produced by reacting nitrogen dioxide with water, see the unbalanced equation below. How many grams of nitrogen dioxide are required to produce 5.00 g of nitric acid?
NO2 + H2O → HNO3 + NO
5.48 g NO2
143.0 miles/hour to m/s (1 mile = 1.609 Km)
63.91 m/s
To the correct number of sig figs, what is the measurement reading on the graduated cylinder?
Cu-63
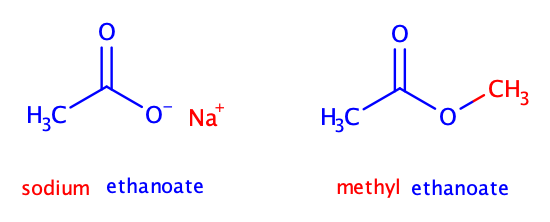 Which of the following has an ester functional group?
Which of the following has an ester functional group?
Which of the following drawings depicts a chemical reaction consistent with Dalton’s atomic theory? (2 answers)
b & d
How many inches long is an imperial star destroyer (7 s.f.)?
(1.6 km long)1 mile = 1.60934 km 1 mile = 5280 ft1 foot = 12 inches
62,992.28 inches
Three students use a 10 mL graduated cylinder to measure 5 mL of methanol for an experiment. Which measurement is most precise? 4.85, 5 or 5.1
4.85
Naturally occurring lithium is a mixture of Li-6 and Li-7. How many protons, neutrons and electrons does each neutral atom have?
Li-6: 3p, 3e, 3n. Li-7: 3p, 3e, 4n
Which of the following organic compounds is an alcohol and which is a carboxylic acid?
CH3CHCH2
CH3CH2COOH
CH3CH2CH2CH2CH2CH3
CHCCH3
CH3CHOHCH3
CH3CHNH2CH3
2 Carboxylic acid
5 Alcohol
If 324.85 g of NaOH and 279.53 g of H2SO4 are reacted to form Na2SO4, which substance is the limiting reagent?
H2SO4
The boiling point of acetone is 132.8°F convert to Kelvin
329.2 K
A piece of wood is first chopped into two pieces. Then each piece is burned. Which of these two processes is a chemical change?
Combustion
Complete table & Identify incorrect isotope
Show table
What is the percent composition of each element in Acetic Acid? (CH3COOH)
C=39.7%, H=6.71%, O=53.3%
What is the percent yield if you did the reaction yourself and produced 324.71 grams?
80.210% yield
A non-existent metal weighs 0.0995 oz. When placed in a graduated cylinder containing water, the liquid rises from 12.72 mL to 19.56 mL. What is the density of the ore in g/cm3 ? (1 oz = 28.3 grams)
4.12*10^-1 g/cm^3
Which of the following drawings represents a collection of H2O molecules? The shaded spheres represent hydrogen atoms and the unshaded represent oxygen atoms
C