Define: Molecule
Formed when two or more atoms bond covalently
T/F: You can put "mono" with the first element
False
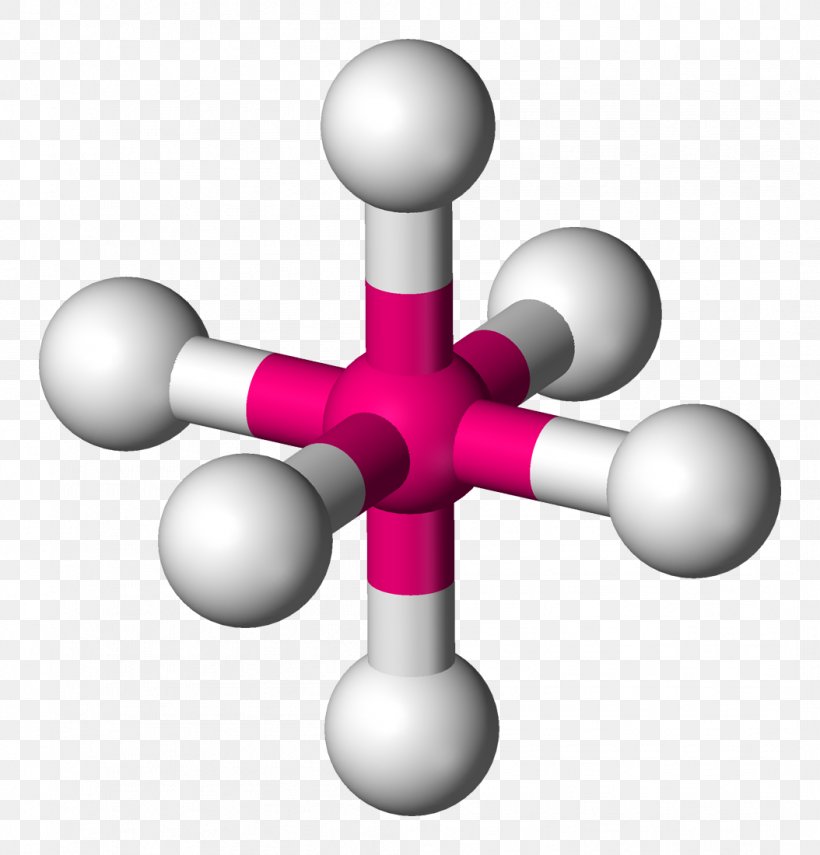
An Octahedral has how many sides?
8
Define: Valence Electron
The electrons in the outermost shell
Draw Bohr model for C-Carbon
p-6
e-6
n-12-6=6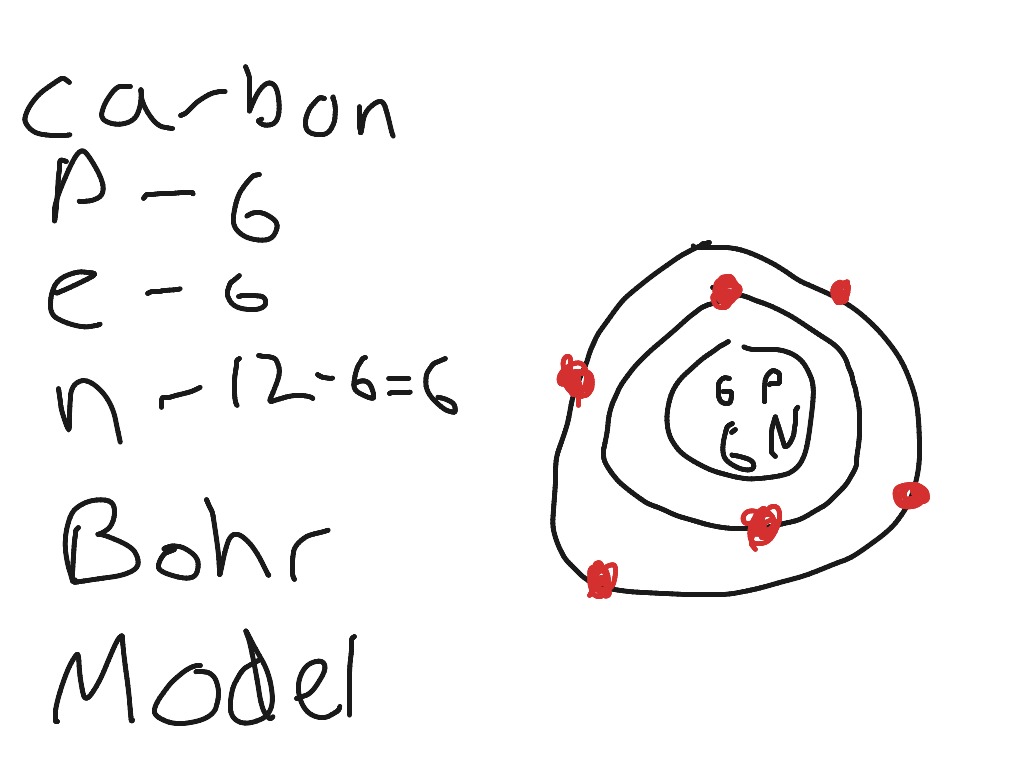
DAILY DOUBLE: Define: Sigma bond and Pi bond
Sigma bond- single covalent bonds
Pi bond- A multiple covalent bond
Molecular Compound name of NH3
Nitrogen trihydride
Lewis Dot Structure for CO2
![]()
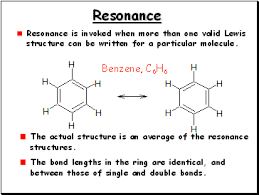
Define: Resonance
How they bond
Electron Configuration of Al(13)

1s22s22p63s23p1
Lewis Structure
represents the arrangement of electrons in a molecule
example: Hydrogen can be written as H-H or H:H
Name:P4S9
TetraPhosphorus DecaSulfide
Anything sp hybridized is...
linear, 180o, electron domain geometry

T/F: Lewis Dot Structures can be unevenly divided while setting up
False must be evenly divided
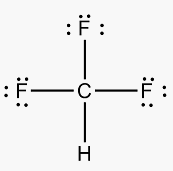
Is the molecule CHF3 polar or nonpolar, and why?
This molecule is polar; the electrons are attracted to one end.
What are unbonded pairs called?
Lone Pairs
Define: Oxyacid
An acid that contains both a hydrogen atom and an oxyanion
NH3 has what type of structural form
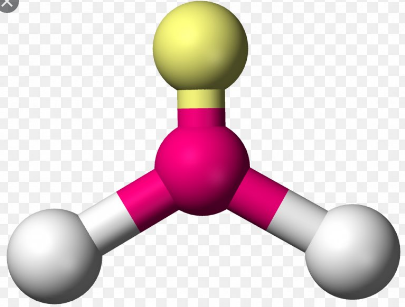
Trigonal Planar
Define: Polar Molecule
Molecule where electrons attract toward one end
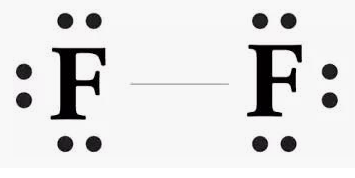
Is the molecule F2 polar or nonpolar, and why?
This molecule is nonpolar; electrons are distributed in the molecule evenly.
-Nonpolar bond-nonpolar molecule
Draw the Lewis Structure of Fluorine
On Board!
binary and oxyacid have what in common?
they both have hydrogen
Define VSEPR
Valance, Shell, Electron, Pair, Repulsion
Define: Dipole Movement
Movement of electrons to one end of molecule
- The end where the electrons left becomes slightly positive
Reason: "dipole"= "di"-2 + "polar" opposites