State of Matter (solid, liquid, gas)... physical or chemical property?
Physical
What is an ionic bond? (Hint: There are 2 parts to an ionic bond that should be mentioned)
A bond formed between a metal & a non-metal where electrons are donated.
The periodic table is organized based off of this pattern, increasing by 1 each element.
Atomic Number (Number of protons).
How many Oxygen are on the products side?
4
Is this balanced?
___ P4 + ___ O2 → ___ P4O10
Nope!
Chemical
Boron and Fluorine bond together... what would the chemical formula be for Boron Fluoride?
BF3
The amount of energy levels corresponds to this part of the periodic table.
Rows/Periods
How many Oxygen are on the products side?
___ Al + ___ O2 → ___ Al2O3
4, 3, 2
Whenever sugar dissolves in water you are observing a _________ property.
Physical
CO2 is a example of a ________ bond
This group of elements is to the left of the staircase on the periodic table.
Metals
Is this equation balanced?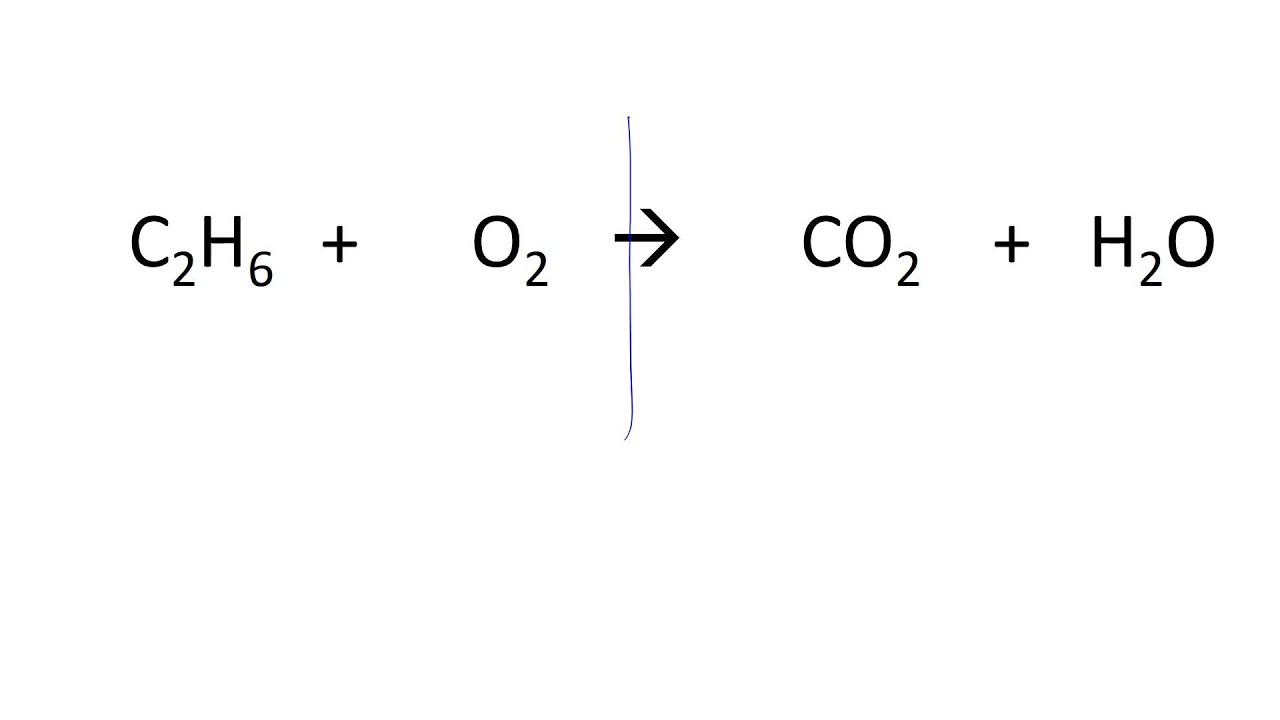
No
___ NaCl + ___ AgNO3 → ___ AgCl + ___ NaNO3
1, 1, 1, 1
The pH scale is a measure if acidity and basicity... which are _______ properties of matter.
Daily Double: Give me an example of an Acid/Base from the pH scale shown in class (400 points).
Chemical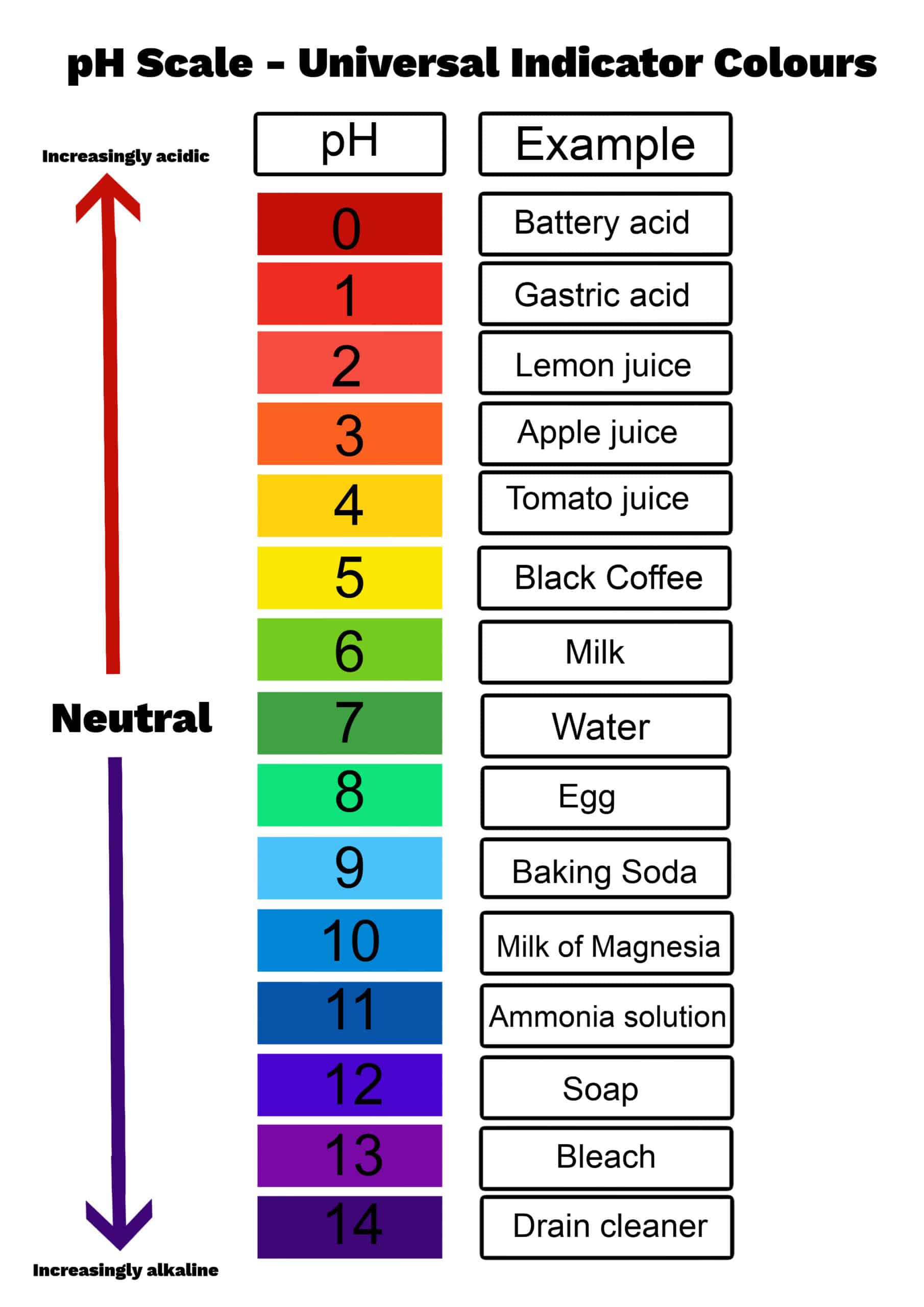
There are two types of ions... what are the name and charge of both?
Cation: Positively charged ion
Anion: Negatively charged ion
What are valence electrons and how many does Carbon have?
Electrons on the outermost energy level of an atom, Carbon has 4 (Group 14).
How many Hydrogen are on the reactants side?
CH3O(CH4)3 + H2O ------> CO2 + OH
17 Hydrogen
___ H3PO4 + ___ KOH → ___ K3PO4 + ___ H2O
1, 3, 1, 3
What is a chemical property?
Something that can only be observed during a chemical change (rearrange atoms to form new substances).
What is a polar covalent bond?
 When electrons are shared, but unequally
When electrons are shared, but unequally
I have 4 energy levels and 4 valence electrons, who am I?
Germanium
Name all of the potential parts of a chemical equation... there should be 6!
___Na + ___H2O = ___NaOH + ___H2
2, 2, 2, 1