Humans are made up mainly of four elements. What are these elements?
Oxygen, Carbon, Hydrogen, and Nitrogen.
On average, how much water does a cell contains?
On average, a typical cell contains between 70% and 75% water by weight.
Water is called this because it can dissolve many ionic and polar substances.
The universal solvent.
What is the logarithmic scale?
A logarithmic scale is a type of scale that increases by powers of 10 instead of by equal steps. In other words, each step on a logarithmic scale represents a tenfold (X10) change in the value being measured (pH).
How many carbon and hydrogen atoms does a molecule of ethane have?
C2H6

What is the atomic number?
The atomic number is the number of protons.
What do you call the bonds that join oxygen and hydrogen in a water molecule?
Polar covalent bonds.
When common salt (sodium chloride, NaCl) is dissolved in water, how do the ions dissociate?
The negatively charged oxygen atoms (-) of water molecules attach to Na ions (+), while the positively charged hydrogen atoms (+) attach to the chlorine ions (-).
What is the pH scale?
The pH scale is a numerical scale used to measure how acidic or basic (alkaline) a solution is.
An alkyne hydrocarbon should have at least one ____.
Triple bond.
What is the atomic mass?
The atomic mass mostly comes from the combined mass of the protons and neutrons in the nucleus.
In a water molecule, is the sharing of electrons equal or unequal?
In a water molecule, the sharing of electrons is unequal. Oxygen attracts electrons more strongly than hydrogen, making the molecule polar.
In solubility terms, cholesterol and water molecules are called....
Hydrophobic
How much more alkaline is soapy water compared to distilled water?
Soapy water is about 100,000 times more alkaline than distilled water.
Soapy water usually has a pH of about 12, while distilled water is neutral, with a pH of 7.
Because the pH scale is logarithmic, each step represents a 10-fold difference in alkalinity or acidity. So, the difference between pH 12 and pH 7 is 5 pH units:
105=100,000What are structural isomers?
Structural isomers are molecules that have the same molecular formula but different arrangements of atoms.
butane: C4H10
Isobutane: C4H10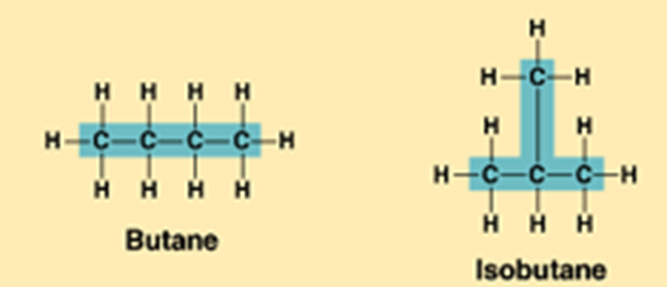
What are isotopes?
Isotopes of an element have the same number of protons but different numbers of neutrons.
What is water polarity?
Water polarity means that a water molecule has a slight positive charge on the hydrogen atoms and a slight negative charge on the oxygen atom, making it a polar molecule. This happens because the electrons are shared unequally between oxygen and hydrogen.
Water molecules interact with each other through ..............., and they can form ................ bonds.
Cohesion and hydrogen
Can water act as an acid and a base?
Water is both an acid and a base.
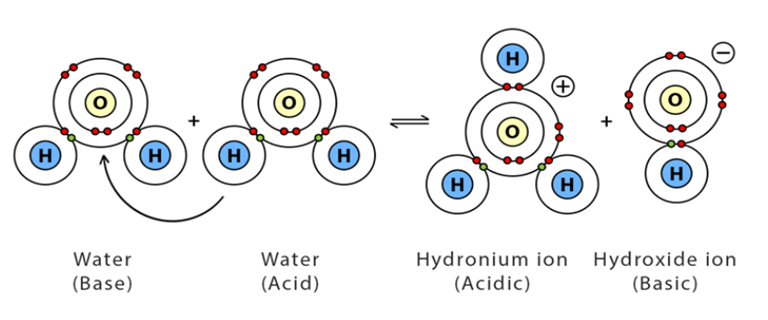
What is the functional group of alcohols?
-OH (hydroxyl group)
How many electron shells do the chemical elements in row five have?
Elements in row 5 of the periodic table have five electron shells.
How do hydrogen bonds form between water molecules?
In water, the slightly positive hydrogen atoms of one molecule are attracted to the slightly negative oxygen atoms of nearby water molecules, forming hydrogen bonds.
Water striders can move along the water's surface because of certain properties of water.
Cohesion and surface tension.
In a beaker of solution X, what does a pH meter measure?
pH measures the concentration of H+.
What is the functional group that plays a key role in the structure of DNA?
The phosphate group (-PO42-)
What do atoms do to complete their outer shell (octet rule)?
Atoms share, gain, or loose electrons to complete their outer shell.
Which is the most accepted theory about the origin of water?
Water was delivered by icy comets and water-rich asteroids during the late heavy bombardment
How do hydrogen bonds affect the boiling and melting points of water?
Hydrogen bonds require extra energy to break, so water has a higher boiling point and melting point than most molecules of similar size.
If you were given HCl with a concentration of 0.005 M, what would be the pH?
pH = -log(5 x 10⁻3)
pH = 2.3
Which is the amino functional group and why is it important?
The amino functional group is -NH2.
It is a key component of amino acids, which are the building blocks of proteins.
The atomic number of oxygen is 8. How many electrons does oxygen need to complete its outer shell?
Oxygen needs two electrons to complete its outer shell.
This term describes the region around a star where conditions are just right for liquid water to exist on a planet’s surface.
The goldilocks zone.
Minerals and nutrients are transported via xylem and phloem vessels in plants. Which properties of water are involved in these processes?
Cohesion, adhesion, capillary action, and transpiration.
What would be the pOH of a solution with a pH of 6.5?
pOH = 7.5 (slightly basic)
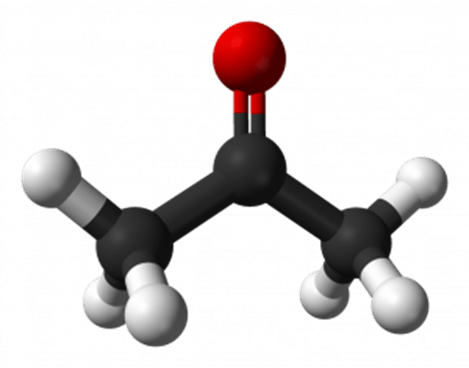
What is the functional group in this compound?
The carbonyl group, C=O (acetone).