What is the name of this element? H
Hydrogen
 The charge of a electron.
The charge of a electron.
What is negative.
The horizontal rows on the periodic 
What are periods.
 Discovered the Periodic table.
Discovered the Periodic table.
Mendeleev.
Good conductors of heat and electricity, shiny, located in the middle and to the left on the periodic table.

Metals.
What is the name of this element? Al
Aluminum
 The charge of a neutron.
The charge of a neutron.
What is neutral.
Vertical columns in the periodic 
What are groups.
 Discovered that electrons follow an orbit.
Discovered that electrons follow an orbit.
Who is Bohr.
Good conductors of electricity, located in the middle of the periodic table, magnesium is an example.

Alkaline Earth Metals.
What is the name of this element? Sn
Tin
Forms when an atom gains or loses 
What is an ion.
Elements are grouped vertically based on____?
What is properties.
 Discovered the proton.
Discovered the proton.
Who is Rutherford.
Less reactive metals, includes iron, nickel, and gold.
:max_bytes(150000):strip_icc()/transitionmetals-56a12cdb5f9b58b7d0bcca90.png)
Transition metals.
Sodium
The small dense center of an 
What is the nucleus.
Elements are in the same group when they are in the same____?
Family.
 Atoms of the same element have the same mass.
Atoms of the same element have the same mass.
Dalton.
Elements in group 18.

Noble gasses.
The atomic number of oxygen?
8
Subatomic particles that are located the farthest away from the nucleus.
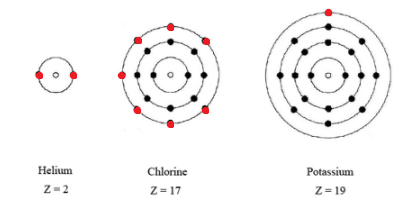
What are valence electrons.
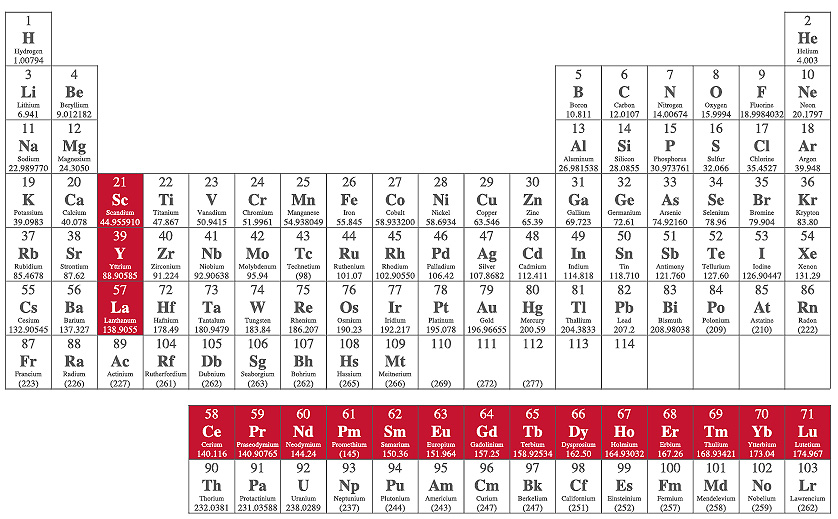 Located on the bottom row of the periodic table, very unstable.
Located on the bottom row of the periodic table, very unstable.
What are Actinides.
 Discovered the electron.
Discovered the electron.
Who is J. J Thomson.
The first period below the periodic table.

Lanthanides.