The chart of elements arranged by properties.
Periodic Table
The name for Group 1A.
Alkali Metals
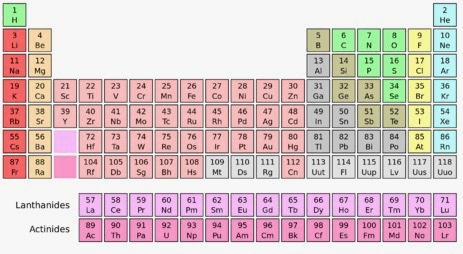 Group 2, Period 3
Group 2, Period 3
Magnesium
 Which represents the reactant in the experiment?
Which represents the reactant in the experiment?
Water
a substance (made up of the same type of atoms) that cannot be chemically broken down any further.
Element
The name for Group 18.
Noble Gases
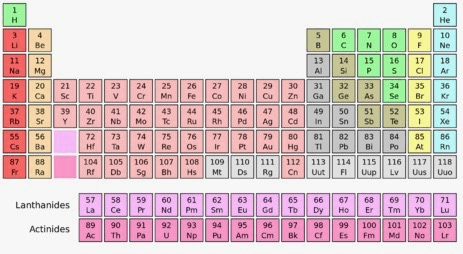 Group 17, Period 2
Group 17, Period 2
Fluorine
The Law that states: mass is neither created nor destroyed in chemical reactions
The Law of Conservation of Mass
The horizontal rows on the periodic table.
Periods
The name for Group 2A
Alkaline Earth Metals
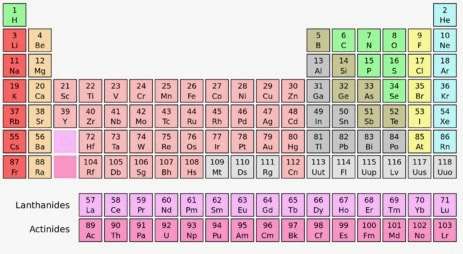 Group 10, Period 4
Group 10, Period 4
Nickel
What law states: "different samples of a pure chemical substance always contain the same proportion of elements by mass"
Law of Definite Proportions
Groups 1, 2, and 13-18 on the periodic table.
Main Groups
Colorful gases that are usually seen in diatomic form.
Halogens
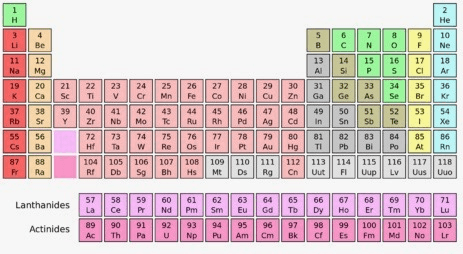 Group 18, Period 2
Group 18, Period 2
Neon
In what experiment was the electron first discovered?
Thomson's Cathode Ray Experiment
The 14 groups shown separately at the bottom of the periodic table.
Colorless gases with low reactivity.
Noble Gases
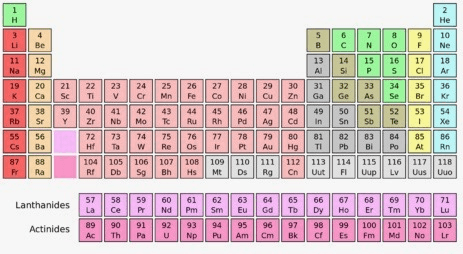 Group 7, Period 4
Group 7, Period 4
Manganese
In what experiment was the charge of an electron discovered?
Millikan's Oil Drop Experiment