Which has greater molecular attraction, isopropyl alcohol (IA) or water?
What is water?
The ______ temperature is a number calculated by adding all numbers together and dividing by how many numbers you added together.
What is average.
Molecules in a hotter sample will be moving _____.
What is faster.
When __________ overcomes the molecular attraction, a phase change can occur.
The air in Mrs. Fullner-Grennen's room measured 16◦C in the morning. Later in the afternoon the thermostat measured 21◦C. What happened to the air molecules during the day?
The molecules started moving faster.
There will always be heat transfer between two objects that are touching if there is a _______ in the temperatures in each object.
What is difference.
An object in its ______ phase do not have enough energy to break the forces of attraction holding them together.
What is solid.
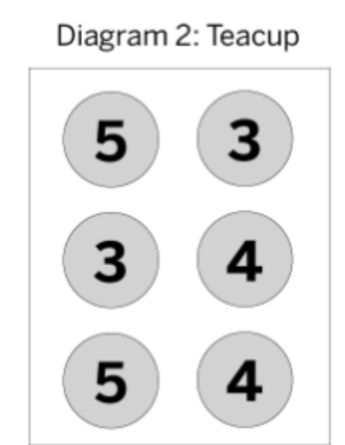
What is 4 and 24.
Heat transfer will stop when ____.
The molecules reach a state of equilibrium
The molecules are moving at the same speed
The kinetic energy is equal
Two different liquids are at room temperature. Scientists cooled them to a lower temperature. Substance 1 changed phase, but Substance 2 did not. What happened to the behavior of the molecules in Substance 1?
Its molecules now move in place forming a solid.
Estimate what final temperature each sample would reach once in contact (this is called equilibrium temperature). Explain your thinking.
40C because energy would transfer from the hotter sample to the colder sample until both reached the same temperature and all molecules are moving at the same pace.
Energy transfer occurs when molecules ______.
What is collide with each other.
Two different liquids are at room temperature. Scientists cooled them to a lower temperature. Substance 1 changed phase, but Substance 2 did not. Substances 1 is different than Substance 2 because _____.
Substance 1 has a stronger attraction between its molecules than Substance 2.
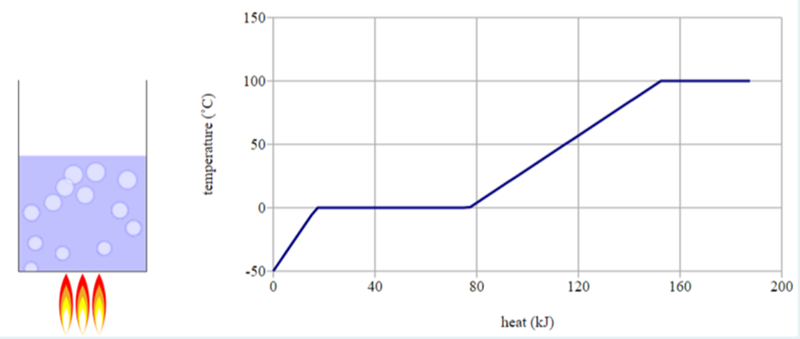
As you increase the temperature of a substance, why does the temperature plateau before increasing again?
This plateau is where a phase change is occuring. For example, water boils at 100F, the water will maintain 100F until all water has evaporated and turned to a gas.