This can be found by adding all of the numbers to get a total and then dividing by the number of numbers that were added.
What is Average?
When a thing gets hotter, it's molecules are moving _____________.
What is faster?
A can of soda has been in a hot car all day. The temperature of the soda has increased, so what has happened to the energy of the molecules of the soda?
What is "the energy/speed has increased"?
Energy is neither created nor destroyed, it transfers.
What is true?
The moment when something hits something else.
What is collision?
This is the type of energy that an object has because it is moving.
What is Kinetic Energy?
Things are made of _____. Water is an example of this term because it has 2 hydrogen atoms joined with 1 oxygen atom.
What is a molecule?
When room temperature water is placed in a refrigerator, it gets colder. Explain what happens.
What is the molecules in the water are moving slower/energy has decreased?
In order for substances to reach equilibrium, both of the objects have to transfer temperatures back and forth.
What is false? (Hot substances transfer to colder)
Temperature is the measure of the _____ speed (kinetic energy) of the molecules of a thing.
What is average?
This is otherwise known as the average kinetic energy of a thing.
What is temperature?
Temperature is a measure of the average ______________ of the molecules of a thing.
What is Kinetic Energy?
Which has more thermal energy, a bathtub full of water at 50C or a small tea cup of water at 100C?
What is the bathtub?
The hotter something is, the slower its molecules move.
What is false? (they move faster)
a) _____ isn't created or destroyed. Therefore, as it transfers, it increases in one part of a system as it b) _____ in another part of the system.
a) What is energy?
b) What is decreases?
The name given to the state of two objects that are at the same temperature.
What is equilibrium?
For things at the same temperature, the thing with ________ molecules has a higher thermal energy.
What is more?
A hot glass of water (Container A) and a cold glass of water (Container B) were placed together. This is what happened to the temperature of each container to reach equilibrium.
What is "the energy flows from Container A to Container B until they reach equilibrium"?
When two substances of different sizes are the same temperature, they have the same thermal energy.
What is false?
The molecules of a system will transfer energy until the system reaches a stable state known as a) _____, in which all molecules are moving at about the b) ____ speed.
a) What is equilibrium?
b) What is same? (which is an average)
This is the rule that states energy can neither be created or destroyed - only transferred
What is Conservation of Energy of the Law of Conversation of Energy?
When two things are in contact, their molecules collide, and a) _____ transfers from the b) _____-moving molecules to the c) _____-moving molecules.
a) What is energy?
b) What is faster?
c) What is slower?
After these two pieces of plastic have been touching for a while, this is the direction of energy transfer.
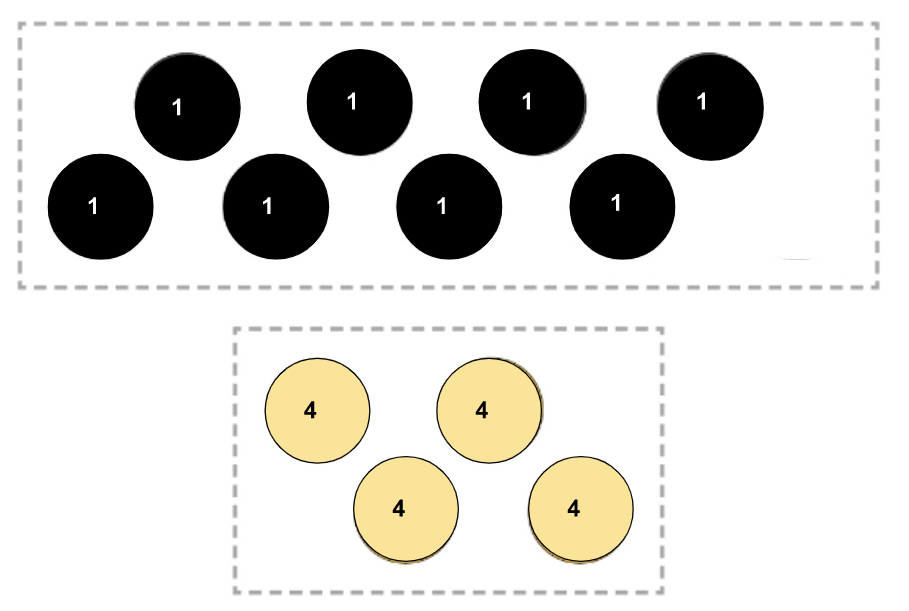
What is from the bottom to the top?
Object A has 500 Kj of energy and object B has 300 Kj of energy. When they touch, energy will transfer from object A to object B.
What is true?
At equilibrium, the a) _____ kinetic energy (temperature) of the molecules in the system is the b) _____ kinetic energy (thermal energy) evenly divided by the number of molecules in the system.
a) What is average?
b) What is total?