The total amount of kinetic energy in a substance
Thermal Energy
When you touch a hot stove and burn your hand, this is an example of what kind of heat transfer?
Conduction
True or False: All molecules are always moving.
True
HOT TO COLD
Distinguish between thermal energy and temperature.
Thermal Energy = total KE
Temperature = average KE
The average of the kinetic energy of a substance
Temperature
The sun transfers energy to Earth's surface through which type of heat transfer?
Radiation
Which of these images represents the molecules of a gas? Explain.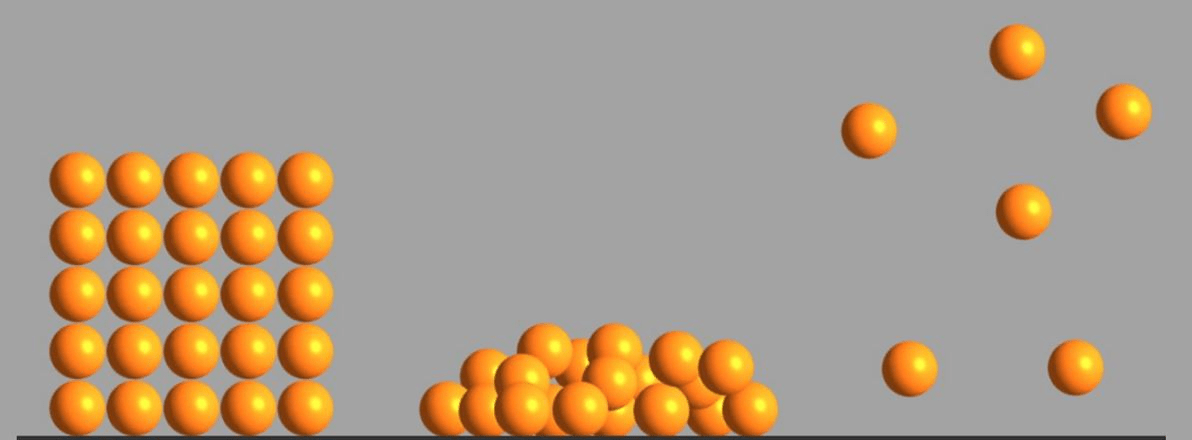
The third image represents a gas because the molecules are moving freely and away from each other.
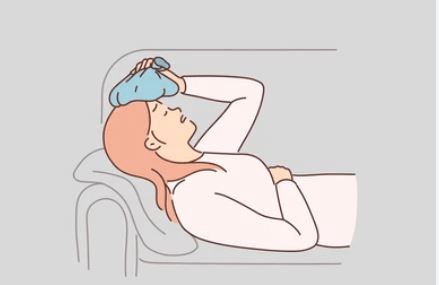 Your teacher has a headache because her students keep shouting "Lebron" all day while she tries to teach. Describe the heat transfer happening between the ice pack and her face.
Your teacher has a headache because her students keep shouting "Lebron" all day while she tries to teach. Describe the heat transfer happening between the ice pack and her face.
Heat energy is transferring from her forehead to the ice pack. This causes her forehead to decrease in temperature while the ice pack increases in temperature. Heat always moves from hot to cold.
Which has more thermal energy: the penguin or the iceberg? Explain.
The iceberg has more thermal energy because it has more molecules (more TOTAL KE).
conductor
A pot of boiling water has molecules that are rising and falling due to different densities. This is an example of what kind of heat transfer?
As a pot of room temperature water is heated up, describe the movement of its molecules and include the words kinetic energy.
The molecules speed up and gain kinetic energy as it is heated up.
Examine this glass of ice water. Describe the heat transfers that will occur if it is left at room temperature for 30 minutes.

Heat will transfer from the air to the water and from the water to the ice cube. This will cause the ice to melt over time. Heat always moves from hot to cold.
Immediately after being scooped out of the big pot of porridge, describe the thermal energy AND temperature of each of these bowls.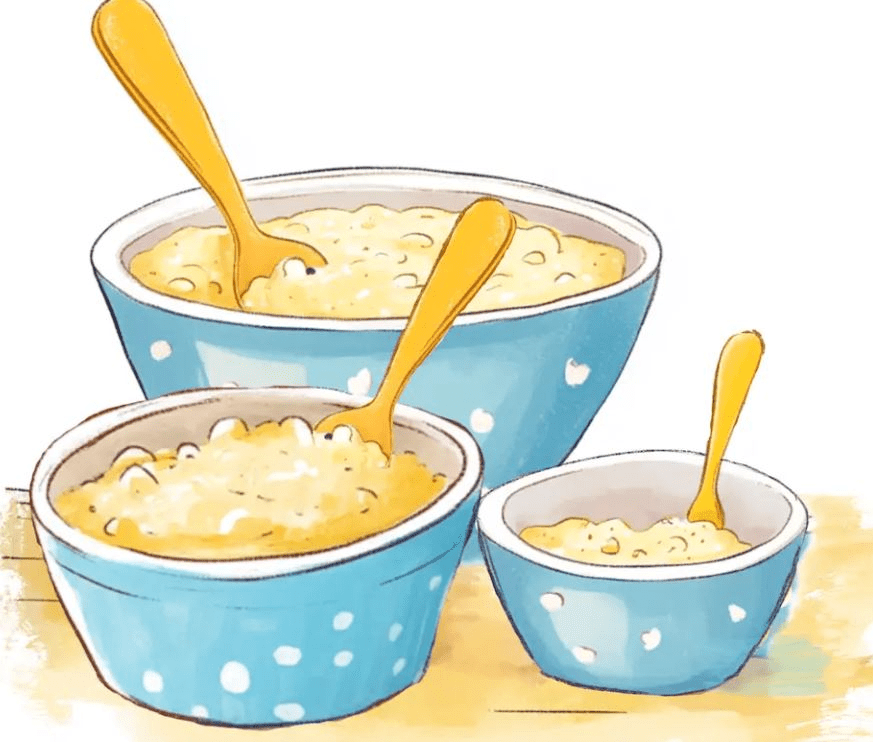
They all start at the same temperature, but the biggest bowl has the most molecules and therefore most thermal energy. Medium bowl has the second most molecules and thermal energy, smallest bowl has the least molecules and least thermal energy.
Something that slows down energy transfer
Insulator
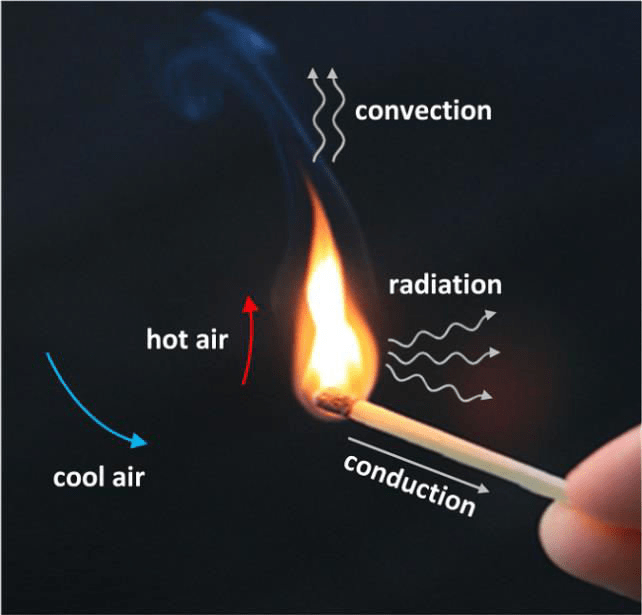
Draw a picture and label examples of all THREE types of heat transfer.
Describe the movement of molecules in a solid, liquid, and gas.
Molecules in a solid are vibrating in place, molecules in a liquid are moving around each other, and molecules in a gas are moving away from each other.
What is wrong with this version of the story? Use at least 2 vocabulary words.
The biggest bowl has the most THERMAL ENERGY since it has the most MOLECULES so it will take the longest for it to cool down. The biggest bowl should have had the highest TEMPERATURE.
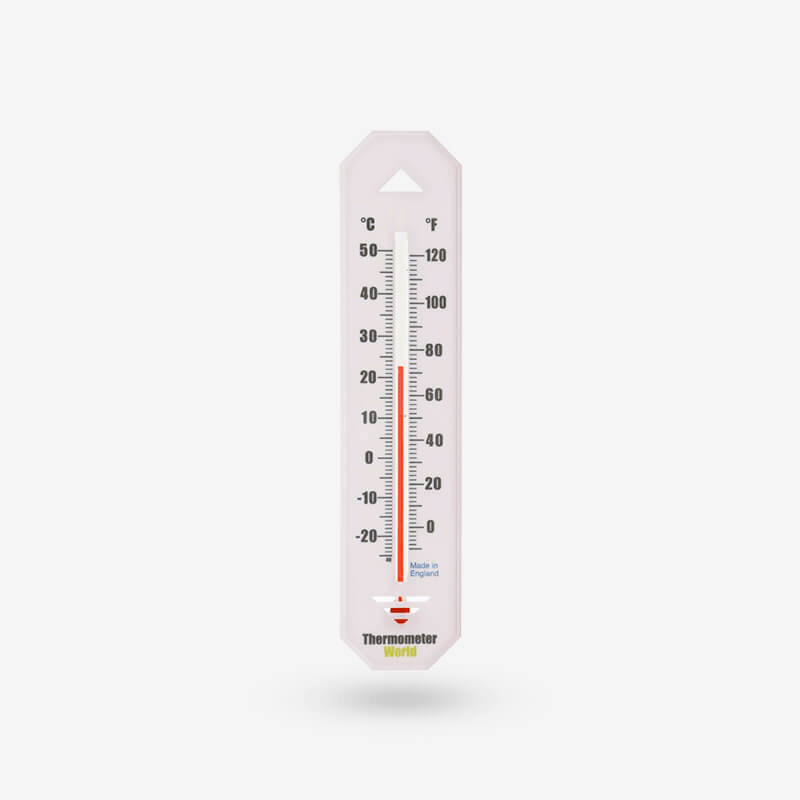 What does a thermometer measure?
What does a thermometer measure?
The average kinetic energy of molecules of a substance
Give the ENTIRE law of conservation of energy.
Energy cannot be created or destroyed but it can change forms.
Use the word "density" to explain what is happening inside of this cup of hot tea.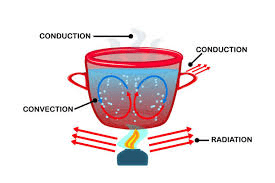
The water is being heated up by the flame. As it is heated, the molecules move apart and it becomes less DENSE and rises up. Further from the heat source, it cools off and molecules move closer together becoming more DENSE and then fall back to the bottom. This pattern repeats.
Draw a model of the hot vs cold water where food coloring was added, including showing how the molecules move in each.
In this model, the arrows represent the SPEED of the molecules.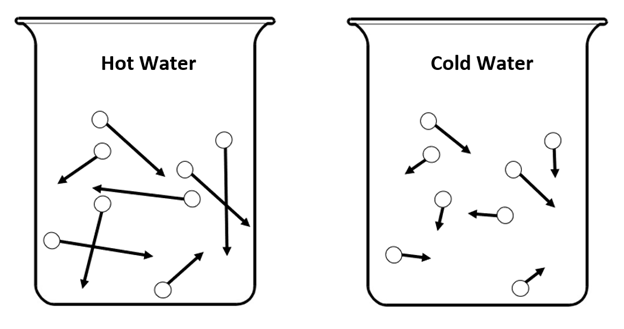
Describe a real life example (involving snow) where heat transfer happened. Use at least 3 vocab words.
Ex: My backpack is about 70 degrees (room temp) and so is the air in the school building. The air in the school building has more thermal energy, because it is made of more molecules than my backpack.