What are the 3 states of matter that we have explored?
Solid, liquid, and gas
What are the particles in a liquid doing?
They are loosely packed and moving around slightly.
Their kinetic energy increases casuing them to move more.
What color is a polar bear's skin?
black
When one object comes in contact with another object it is called a ...
collision
The process of a liquid turning into a gas is called what?
Evaporation
Draw a diagram of particles in a solid and how they behave.
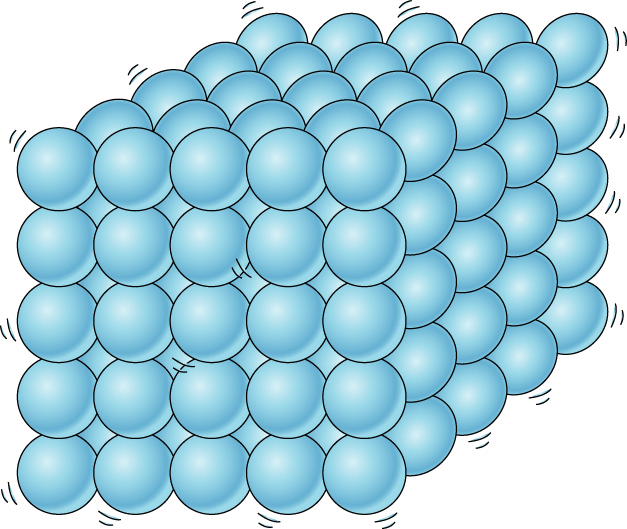
True or False:
Particles with more kinetic energy transfer energy to particles with less kinetic energy.
True.
If you throw a blue stone into the Red Sea, what does it become?
wet
The speed of one object can only be changed if energy is __________ to or from that object.
transferred
The process of a gas turning into a liquid is called what?
Condensation
As particles collide in a material, they __________ kinetic energy to the next particle.
transfer
Materials made up of more particles require more ________________ to increase their temperature.
kinetic energy
What is Mr. Clark's daughter's name?
Rosalie
_________ energy is energy in motion, while _________ energy is energy at rest.
kinetic, potential
Which state of matter has NO definite shape, and NO definite volume?
A gas.
True or False
All particles in a material move with the same kinetic energy.
False
Temperature is actually a measurement of the average _______________ in a system.
kinetic energy
What year will you all graduate high school?
2030
A roller coaster moves down a track, does not collide with another object but eventually slows down. This can be explained by on or both of the following forces.
air resistance, friction
Which state of matter has the most kinetic energy on average? Why?
A gas, becsaue the particles are moving more freely and quickly
What is the relationship between the kinetic energy that particles have and the temperature of a material?
The greater the average kinetic energy of particles the greater the temperature.
Temperature changes when energy moves from ___________ to __________ matter.
warmer, cooler
When creating lemon soda, we mixed different materials together and created something totally different. This is an example of a...
chemical reaction
All materials have __________ to some degree and can go back to its original shape. However eventually everything reaches it's ______________ and cannot return to its original form.
elasticity, breaking point