In which sample are the molecules moving faster?
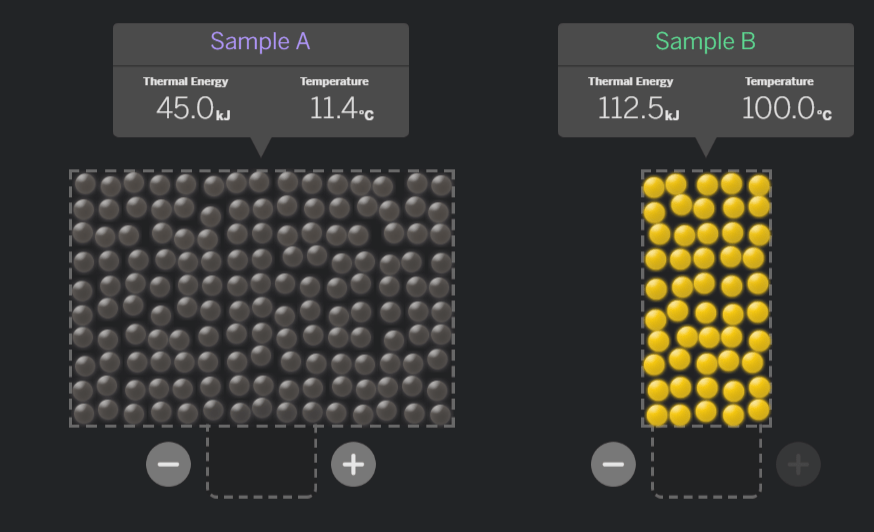
Sample B.
(The temperature is higher, which means the molecules are moving faster.)
Is this system at equilibrium?
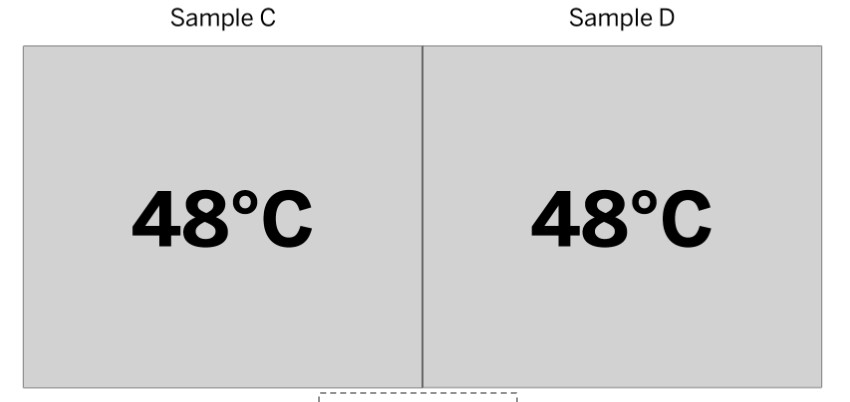
Yes
What other grade does Ms. Stypeck teach?
8th grade
A gallon of milk is in the refrigerator at 40 degrees F.
A half gallon of apple juice is in the same refrigerator.
Which drink has a higher thermal energy?
Gallon of milk
(It's bigger! Both drinks are the same temperature, so the bigger one has more thermal energy.)
Energy transfers from...
A) higher energy to lower energy
B) lower energy to higher energy
C) hotter energy to colder energy
D) colder energy to hotter energy
E) This is a trick question. Energy does not transfer.
A) higher energy to lower energy
These two cubes are the SAME size. How much energy will each cube have when they reach equilibrium?
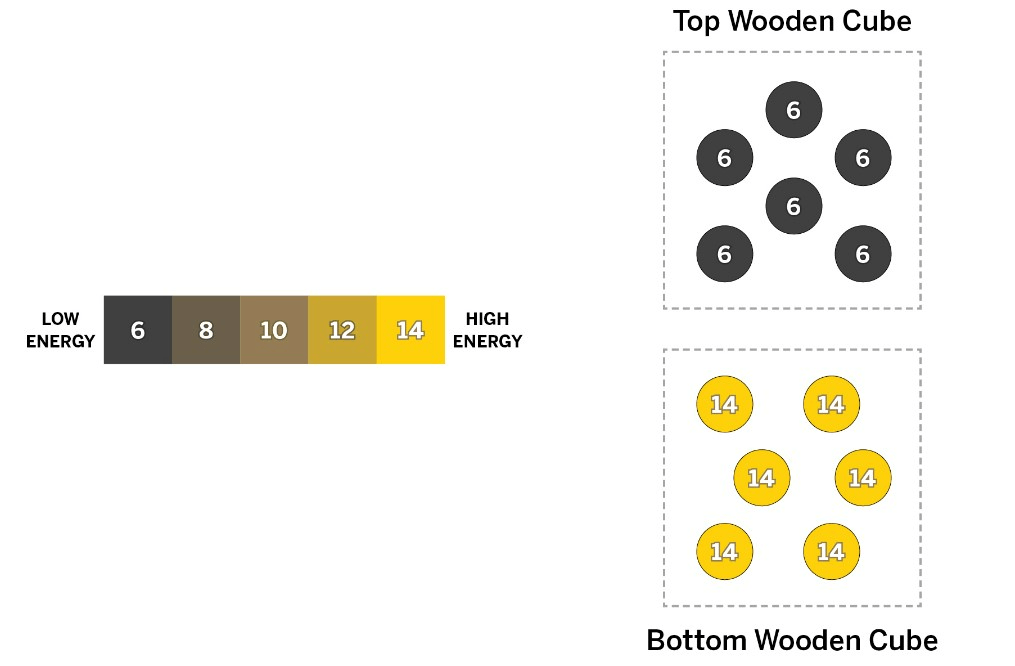
10
Which one of Ms. Stypeck's cat weighs more: Bert or Tibbs?
Bert
A small pizza and a large pizza are put in the freezer which is at 5 degrees Fahrenheit for 1 week.
1. Which pizza is colder?
2. Which pizza has more thermal energy?
You must answer both questions for the points.
1) both pizzas are the same temperature
2) the large pizza has more thermal energy
Lava is hot liquid rock. When lava flows over solid ground, the solid ground beneath it increases in temperature. What happens to the molecules in the solid ground when the temperature is increasing?
They gain energy; they start moving faster.
The numbers inside the circles represent how much energy the molecule has. Is this system at equilibrium?
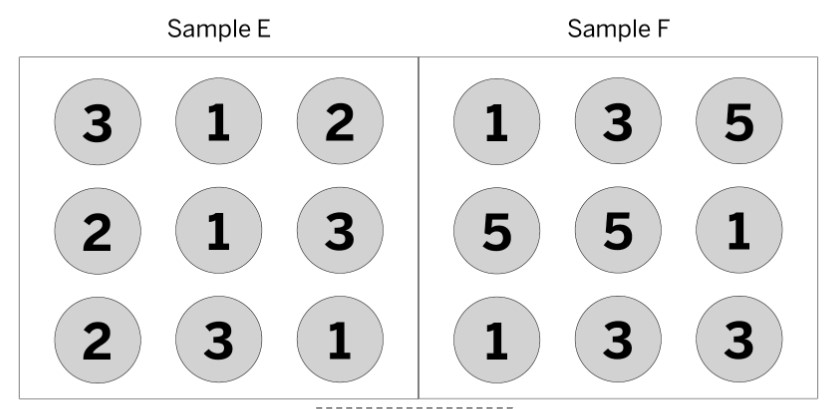
No
Which one of Ms. Stypeck's cat is older: Bert or Tibbs?
Tibbs
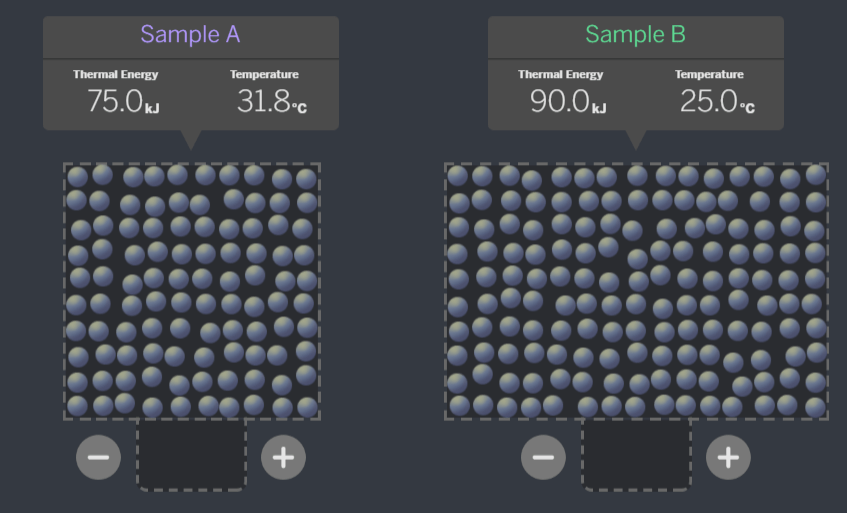
The total thermal energy of this system (Sample A + Sample B) is 157.5 kJ.
When the samples are brought together, will the thermal energy of the system change?

No!
Kinetic energy transfers between molecules, but the thermal energy of the whole system (Sample A + Sample B) will stay the same. It is just redistributed between the samples!
A iceberg has a thermal energy of 315 kJ.
A cup of tea has a thermal energy of 57.6kJ.
What direction will the kinetic energy of the molecules transfer if Ms. Stypeck pours her cup of tea on iceberg?
tea ---> iceberg.
Thermal energy doesn't matter! The tea is still hotter, molecules moving faster. There is just less of them.
Kinetic energy always transfers from more to less
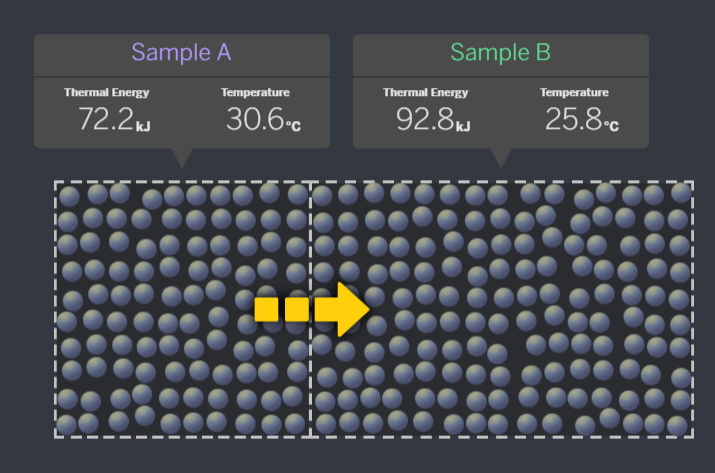
When these samples are brought together, how will energy transfer? A to B or B to A?
A to B
Sample A has a higher temperature. Kinetic Energy will always transfer from the hotter sample to the colder sample, until they are at equilibrium.
What other Seattle high schools has Ms. Stypeck worked at? (**hint there are two!)
University Prep and Lincoln High School
After the samples make contact and reach equilibrium, which system will have a higher temperature?
System A
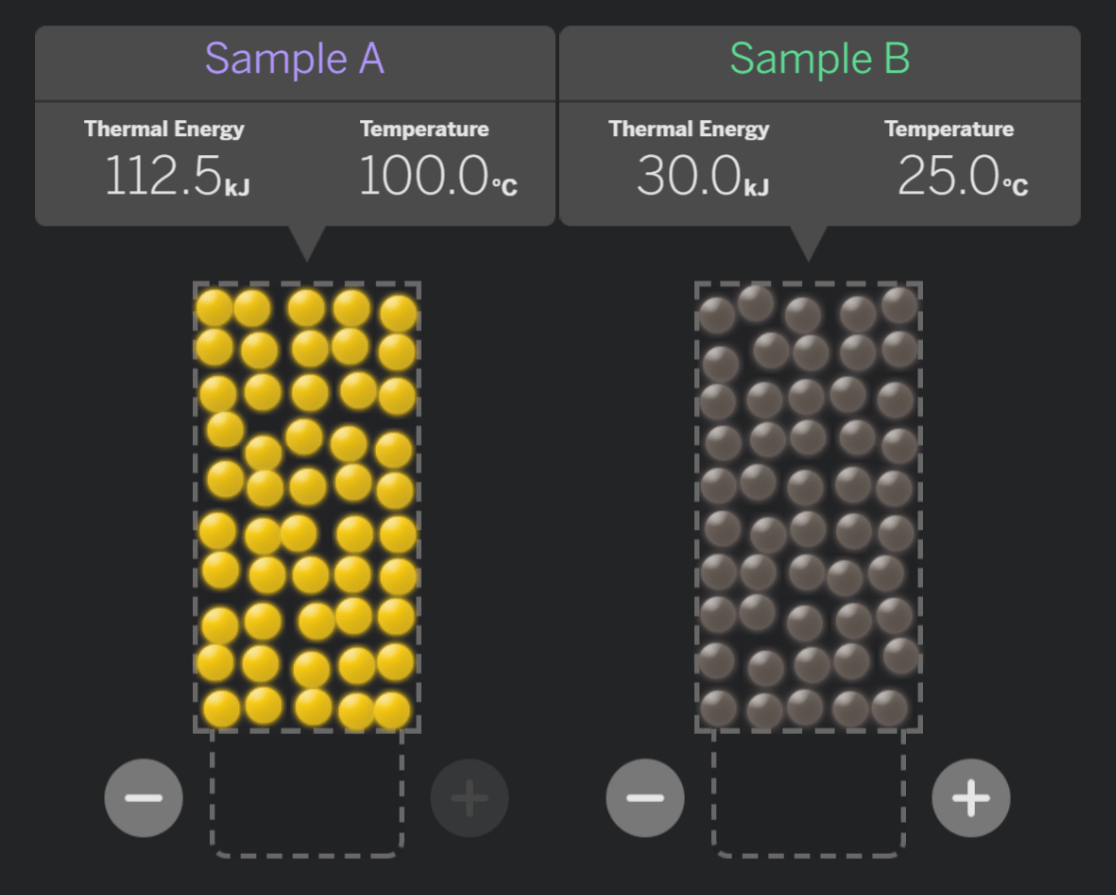
System B
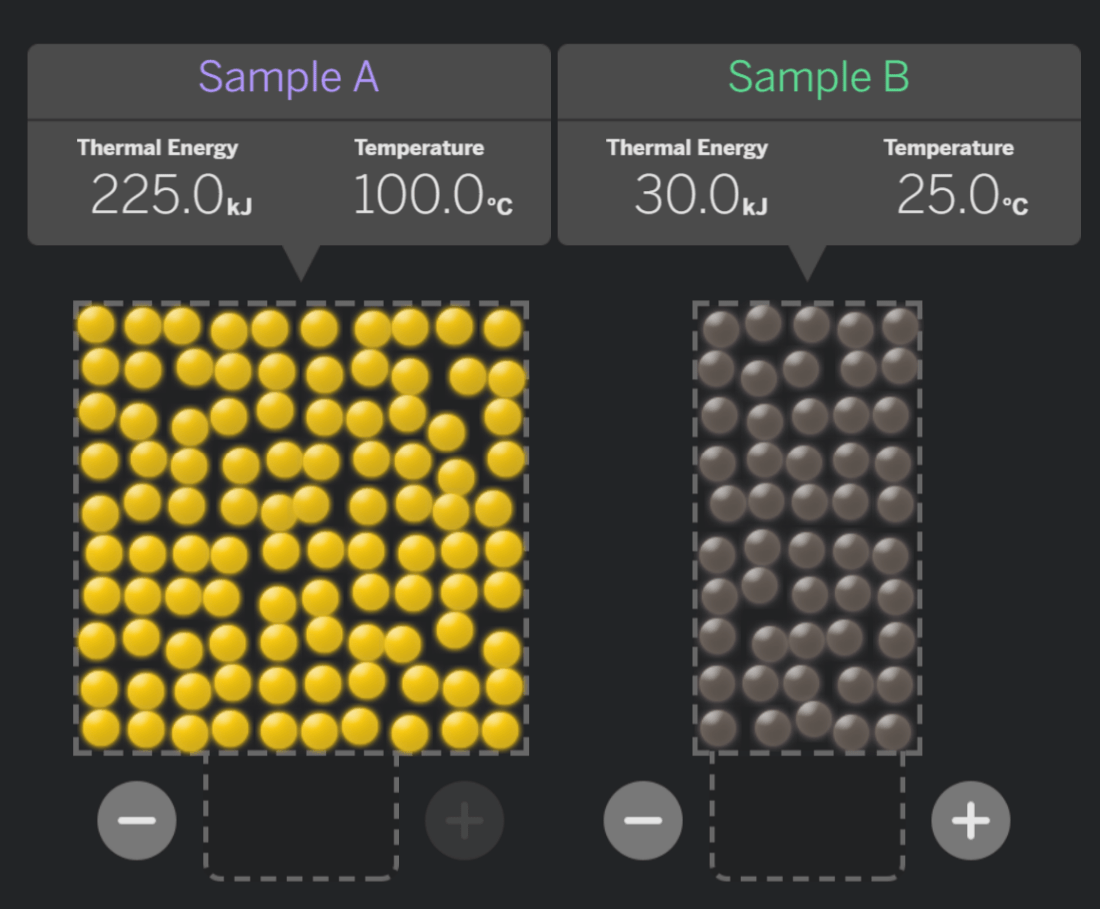
System B
Below is an image from the Thermal Energy SIM. At what point do you think the person running the simulation made Samples A and Sample B collide?
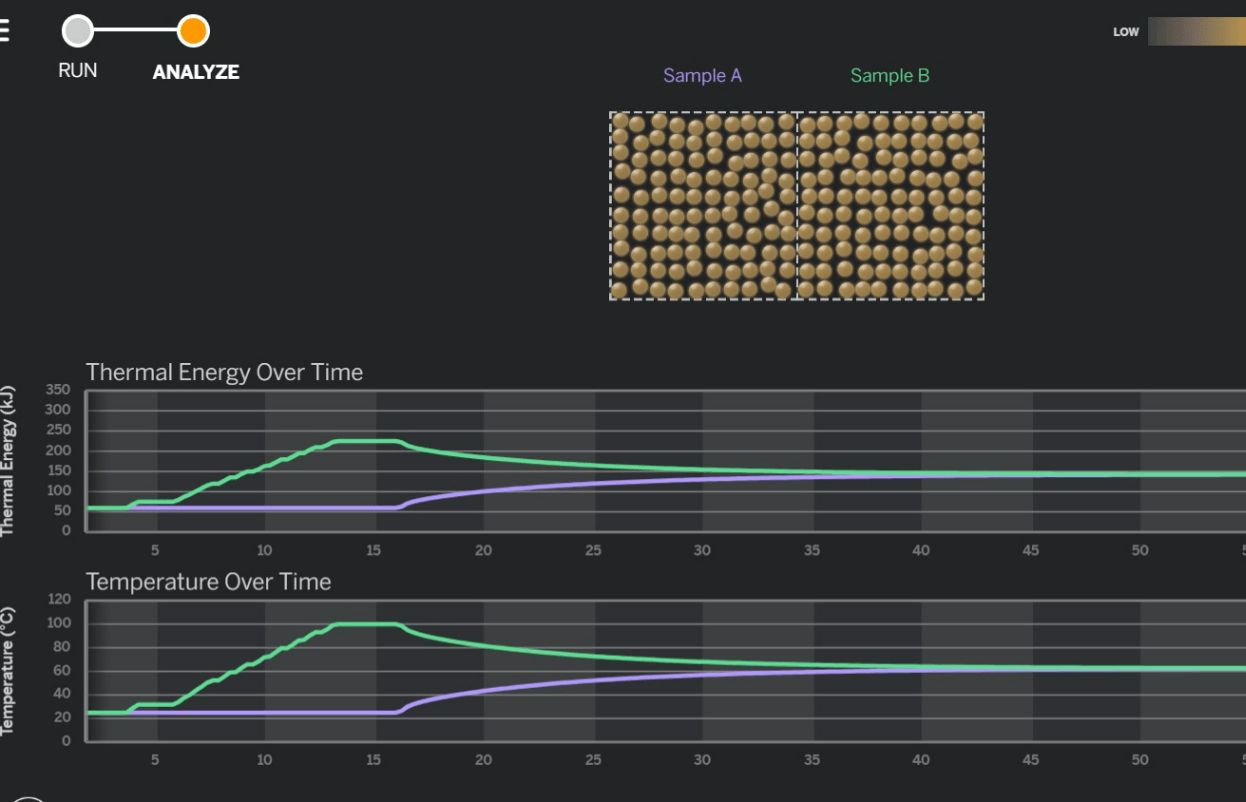
A) Between 0 and 5 seconds
B) Between 15 and 20 seconds
C) Between 25 and 30 seconds
D) Between 35 and 40 seconds
B) Between 15 and 20 seconds
Which of these systems, if the samples were brought together, is ALREADY at equilibrium?
A: 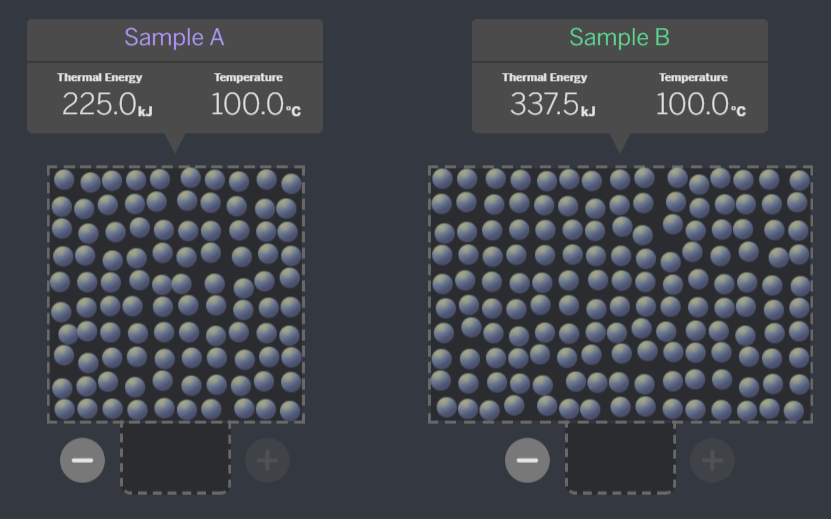
B: 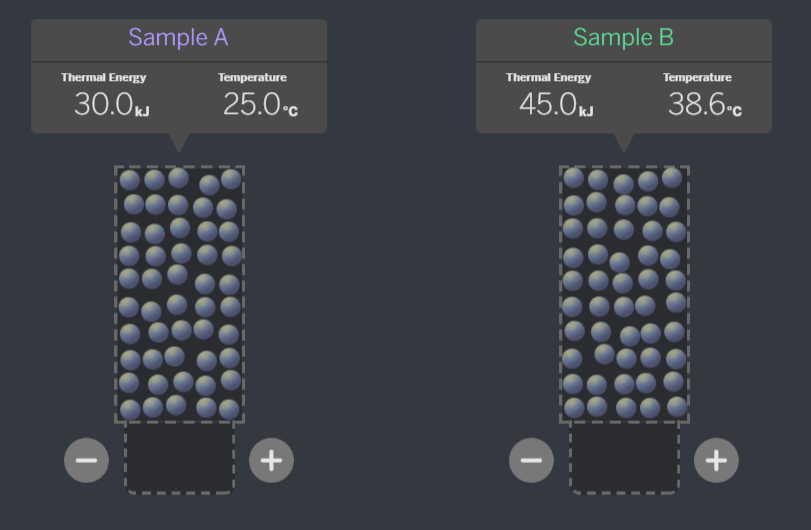
C: 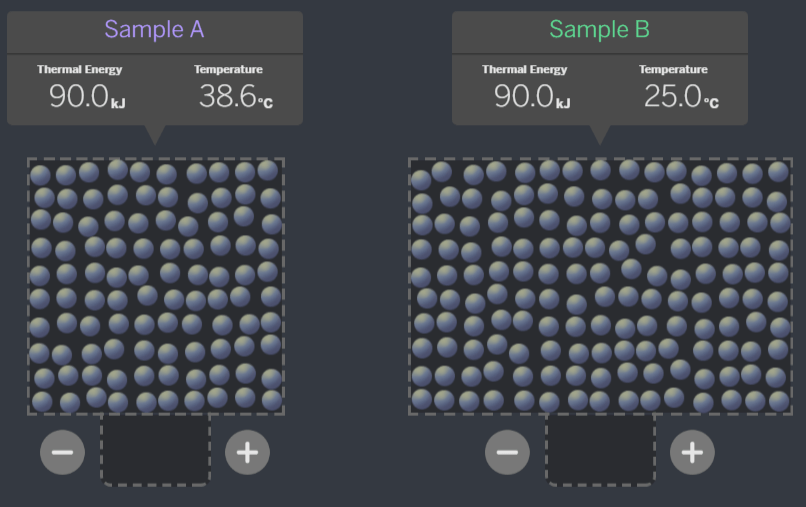
A - When the temperatures are equal, they are at equilibrium. If the molecules are all moving the same speed, they will not transfer energy when they collide.
Name all 4 Science Teachers at McClure.
Mr. Bishop
Mr. McKean
Ms. Stypeck
Ms. Goodell
Peter Pencil states "Temperature and Thermal Energy are the EXACT same because they both are determined by the amount of Kinetic Energy an object has."
Why is he incorrect?
Temperature is the AVERAGE kinetic energy of the molecules in a sample.
Thermal Energy is the TOTAL kinetic energy of the molecules in a sample.