When looking at a thermometer, how do we know heat has been absorbed? How do we know if heat has been lost?
Heat absorbed- Temperature rises.
Heat lost- Temperature decreases
When temperature decreases, what happens to the kinetic energy?
Kinetic energy also decreases (the molecules slow down)
Identify Conduction and Give 1 Example
Heat transfer from touch
Ex- Spoon in a hot Mug
Can Conduction work from a liquid to a solid?
Yes! As long as it touches
When copper is added to water, thermal energy between the copper and the water is transferred via what? (Conduction, Convection, Radioation)
Conduction
How would you increase the average kinetic energy of particles within a glass of water?
Heat it up! Microwave, stove, oven.
Identify Convection and Give 1 Example
Convection- Heat rises, cold sinks
Ex. Heated air in a room circulates
At what time did the molecules in the water reach their maximum level of kinetic energy?
6
Concerning Conduction, what is a way to increase the rate of heat transfer?
Increase the interaction area (area being touched)
Higher kinetic energy means what for temperature and movement?
Higher temperature and movement
Identify Radiation and Give 1 Example
Radiation- Light and electromagnetic waves
Ex- sunlight and microwave
Identify if the letters are increasing or decreasing in their Kinetic energy
A and C are increasing
B and C are Decreasing
1. Convection
2. Conduction
3. Radiation
Explain how a state of matter change is connected to kinetic energy and temperature.
The more kinetic energy, the higher the state of matter. More kinetic energy is gas. Low kinetic energy is solid
When a coffee is left in a car overnight, what will happen to the kinetic energy/ temperature?
It will decrease and come to a balance (equilibrium)
Who has the higher kinetic energy? Temperature?
A has higher kinetic energy and temperature
He timed how long it took each ice cube to melt and the water to reach room temperature, and found that the ice in the __________ melted the fastest. This is because there are _________ molecules that transfer thermal energy into the ice through __________.
water, more, conduction
He timed how long it took each ice cube to melt and the water to reach room temperature, and found that the ice in the __________ melted the fastest. This is because there are _________ molecules that transfer thermal energy into the ice through __________.
Water, more, Conduction
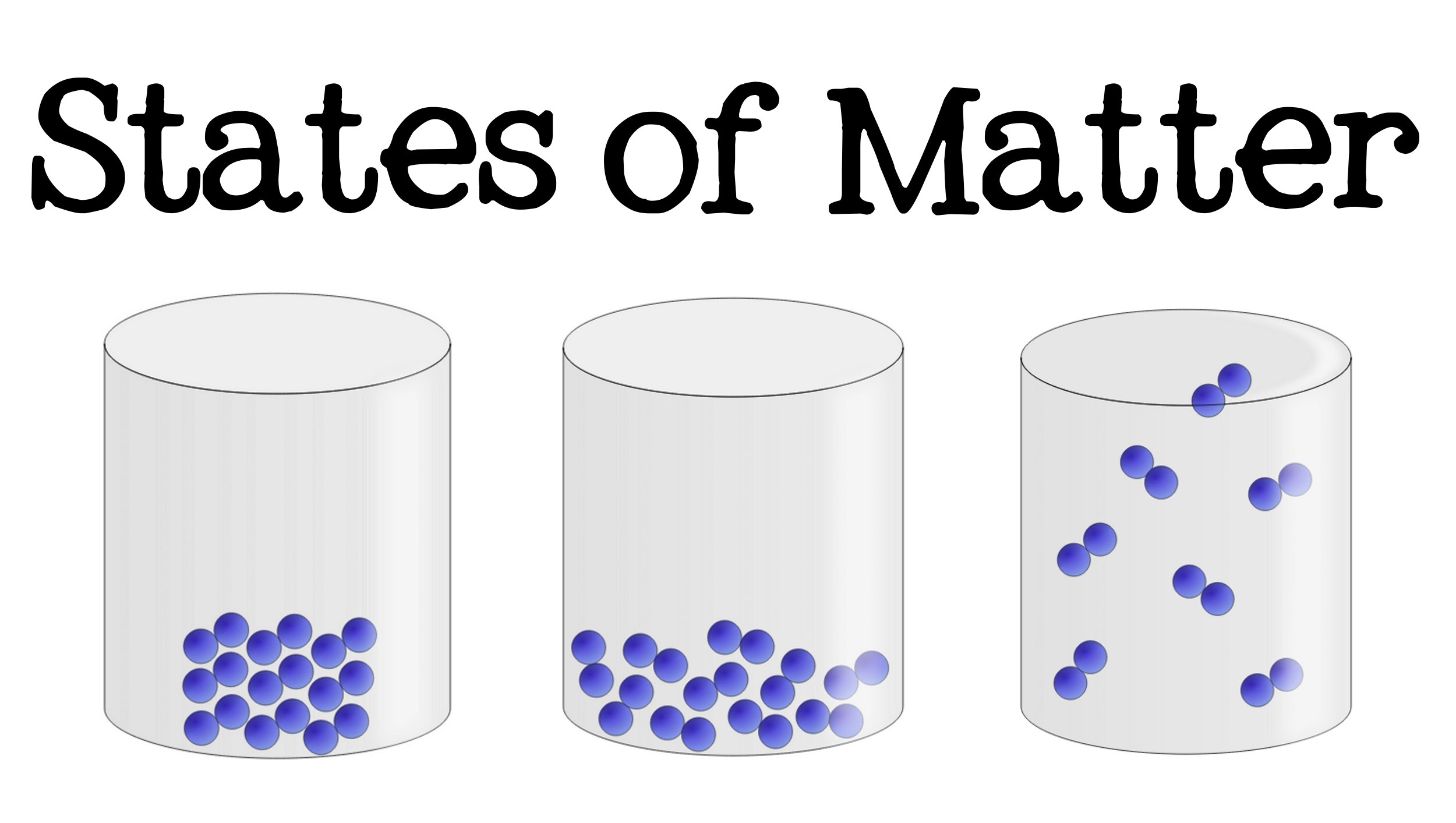
Solid, Liquid, Gas
What was Mr. Ayala's favorite dish in Peru?
Peruvian Mashed Potatoes - Causa
