This is the study of energy changes that occur during chemical and physical changes.
Thermochemistry
A sample of peanut is combusted directly below a can containing 250.0 g of water initially at 24.0 degrees Celsius. The reaction releases 4.2 kJ of heat energy, all of which is transferred to the water. What is the final temperature of the water? (The specific heat of water is 4.2 J/(gxC).)
28.0 degrees Celsius
Hydrogen peroxide, which is often used to disinfect tools and clean surfaces, can decompose to water and oxygen by the reaction below. Is the reaction endothermic or exothermic? Calculate the quantity of heat released when 5.00 g of liquid hydrogen peroxide decomposes at constant pressure. Enthalpy = -196 kJ/mol
exothermic
-14.4 kJ
or
14.4 kJ are released
Ice melting to form liquid water is an example of
a. An exothermic process because it releases heat
b. An endothermic process because it releases heat
c. An exothermic process because it absorbs heat
d. An endothermic process because it absorbs heat
d. An endothermic process because it absorbs heat
Riddle: I can be cracked. And I can be made. I can be told. And I can be played. What am I?
a joke
The ______ of a substance is the amount of heat needed to raise the temperature of 1 gram of the substance by 1 degree Celsius
specific heat capacity
When 27.0 g of an unknown metal at 88.4 °C is placed in 115 g H2O at 21.0°C, the final temperature of the water is 23.7°C. What is the specific heat capacity of the metal? The specific heat capacity of water is 4.184 J/g·K.
0.740 J/g·K
The gas-phase decomposition of iodoethane, C2H5I(g), is represented above. The bond enthalpies for the bonds broken and formed in the reaction are shown below. Determine the enthalpy of the decomposition reaction.
87 kJ/mol
2Ca(s) + O2(g) --> 2CaO(s) ΔH = x
Ca(s) + CO2(g) + 1/2O2(g) --> CaCO3(s) ΔH = y
Based on the information in the table below, which of the following expressions is equal to ΔH rxn for the reaction represented below?
CaCO3(s) --> CaO(s) + CO2(g)
a.) x/2 + y
b.) x/2 - y
c.) x + 2y
d.) x - 2y
b.) x/2 - y
What is a group of owls called?
a. an army
b. a bewilderment
c. a parliament
d. a surprise
c. a parliament

What is shown in the diagram below?
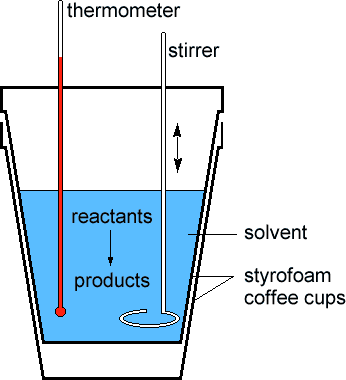
coffee cup calorimeter
C2H5OH(l) + 3O2(g) --> 2CO2(g) + 3H2O(l)
Enthalpy of reaction = -1367 kJ/molrxn
Ethanol, C2H5OH(l) (molar mass 46.1 g/mol), has been proposed as an alternative to traditional cooking fuels. The combustion of C2H5OH(l) is represented by the equation above. Calculate the mass of C2H5OH(l) that would need to be combusted to heat 250.0 g of water from 20.0 degrees Celsius to 100.0 degrees Celsius. (Assume that all of the heat released by the reaction is absorbed by the water and that the specific heat of water is 4.184 J/(gxC).)
2.82 g
The bond enthalpies of the N≡N and F-F bonds are 945 kJ/mol and 159 kJ/mol, respectively. Based on the value of 𝛥Hrxn for the reaction represented below, what is the average bond enthalpy (in kJ/mol) of a N-F bond in NF3?
N2(g) + 3 F2 → 2NF3(g) 𝛥Hrxn = -264 kJ/mol
281 kJ/mol
Cl2(g) + F2(g) --> 2ClF(g)
Based on the bond enthalpies given in the table below, what is the value of ΔH rxn for the reaction above?
a. -140 kJ/mol
b. -120 kJ/mol
c. 120 kJ/mol
d. 140 kJ/mol
b. -120 kJ/mol
Which two mammals lay eggs?
The echidna and the platypus.


The heat absorbed or released from a system under constant pressure is known as this.
Enthalpy
A student mixes 50.0 mL of 1.0 M HCl(aq) and 50.0 mL of 1.0 M NaOH(aq), both at 21.0°C, in a coffee-cup calorimeter. The temperature of the resultant solution increases from 21.0°C to 27.5°C. The products are liquid water and aqueous sodium chloride. Calculate the enthalpy change for the reaction in kJ/mol HCl, assuming that the calorimeter loses only a negligible (so small or unimportant as to be not worth considering) quantity of heat, tha the total volume of the solution is 100.0 mL, that its density is 1.0 g/mL, and that its specific heat is 4.18 J/(g°C).
-54 kJ/mol HCl
Solve for the enthalpy of reaction below.
-304.1 kJ
A 15.0 g silver block is heated to a temperature T1 and then dropped into 15.0 g of water at a lower temperature T2 in a polystyrene cup. Which of the following is true of the final temperature of the system when thermal equilibrium is reached?
a. The final temperature is closer to T1 than to T2
b. The final temperature is exactly halfway between T1 and T2
c. The final temperature is closer to T2 than to T1
d. The final temperature cannot be determined without knowing T1 and T2
c. The final temperature is closer to T2 than to T1
What city is famous because it was destroyed in 79 AD when a nearby volcano, Mount Vesuvius, erupted, covering it in at least 6 meters of ash and other volcanic debris?
Pompeii
This states the total enthalpy change during the complete course of a chemical reaction is independent of the sequence of steps taken.
Hess's Law
When aqueous lead (II) nitrate and aqueous sodium sulfide react, solid lead (II) sulfide and aqueous sodium nitrate are formed. When a 50.0 mL sample of 0.20 M aqueous lead (II) nitrate and a 50.0 mL sample of 0.20 M aqueous sodium sulfide are mixed in a calorimeter, a reaction occurs. If the temperature of the mixture increases from 23.0C to 26.0C, what is the experimental value of ΔH rxn in kJ/mol Pb(NO3)2?
-130 kJ/mol
Provide a real world example of technology that takes advantage of water's high specific heat capacity.
Ex - water can be used to harness the Sun’s energy. Solar radiation beats water, which is circulated in buildings to provide heat.
Name the song.
Dance Monkey