The energy change that accompanies a chemical or physical change.
First Law of Thermodynamics
Energy cannot be created or destroyed. It can only change form.
A reaction is determined to be spontaneous at 25 degrees C. Does that mean it will automatically happen when you mix the reactants at 25 degrees C?
Nope
You and a friend are going to a store. You each leave at the same time and take a different route to get there. You arrive before your friend. Which of the following is a state function: the path you traveled, the time it took you to get there, or your destination?
The destination is the state of function :)
Bond energy
The energy required to break a mole of a given type of bond.
Entropy
A measure of the amount of energy in a system that is not available to do work.
A reaction has a positive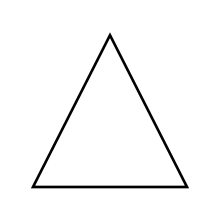 H. Is it exothermic or endothermic?
H. Is it exothermic or endothermic?
The reaction is endothermic.
A physical process in a system cools its surroundings and is spontaneous. Does it result in an increase of decrease of entropy for the system.
Increase
State Function
A property that is independent of path.
Second Law of thermodynamics
The entropy of the universe can never decrease. it must always stay the same or increase.
What is the standard enthalpy of formation of Fe (s)?
0
A reaction cools the flask that is holding it. What is the sign of the reaction's 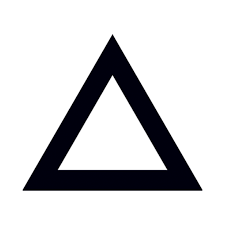 H?
H?
Positive
The energy required to initiate a chemical reaction.
Choose the option that has more entropy: A vase sitting on a shelf or that same vase broken into many peaces.
The vase broken into many peaces
Choose the option that has more entropy: A bunch of confetti scattered evenly on the floor of a three meter by three meter room or the same amount of confetti scattered evenly on the floor of a two meter by two meter room.
Thermodynamics
The study of the relationships and conversions between different forms of energy.
Choose the option that has more entropy: A sample of water as a liquid at 0 degrees C or the same mass of water as an ice cube at 0 degrees C.
Sample of water
Choose the option that has more entropy: A copper pipe that is cold or the same copper pipe that is hot?
The hot pipe :)