 Greek philosopher around 430 B.C who theorized that all matter was made up of invisible particles called atoms. Unfortunately, his theory was not believed.
Greek philosopher around 430 B.C who theorized that all matter was made up of invisible particles called atoms. Unfortunately, his theory was not believed.
Democritus
What charge does a Proton, Neutron and Electron have?
Proton = Positive
Neutron = Neutral
Electron = Negative
Valence Electrons for Chlorine. Chlorine has 17 total Electrons.
7 Valence Electrons
An atom has gained one or more electrons
Negative Ion
Rain is an example of an Acid or Base
Acid
 A very popular Greek Philosopher that believed that all matter is composed of the four elements; Earth, Water, Air, and Fire.
A very popular Greek Philosopher that believed that all matter is composed of the four elements; Earth, Water, Air, and Fire.
Aristotle
According to the formula that we use with the Electron Cloud Diagram, 2n2. How many maximum electrons can the fourth layer of electrons hold?
32
We use the ________ to show the element symbol and dots to represent outer energy level electrons.
An atom has lost one or more electrons
Positive Ion
Vinegar is an example of an
Acid
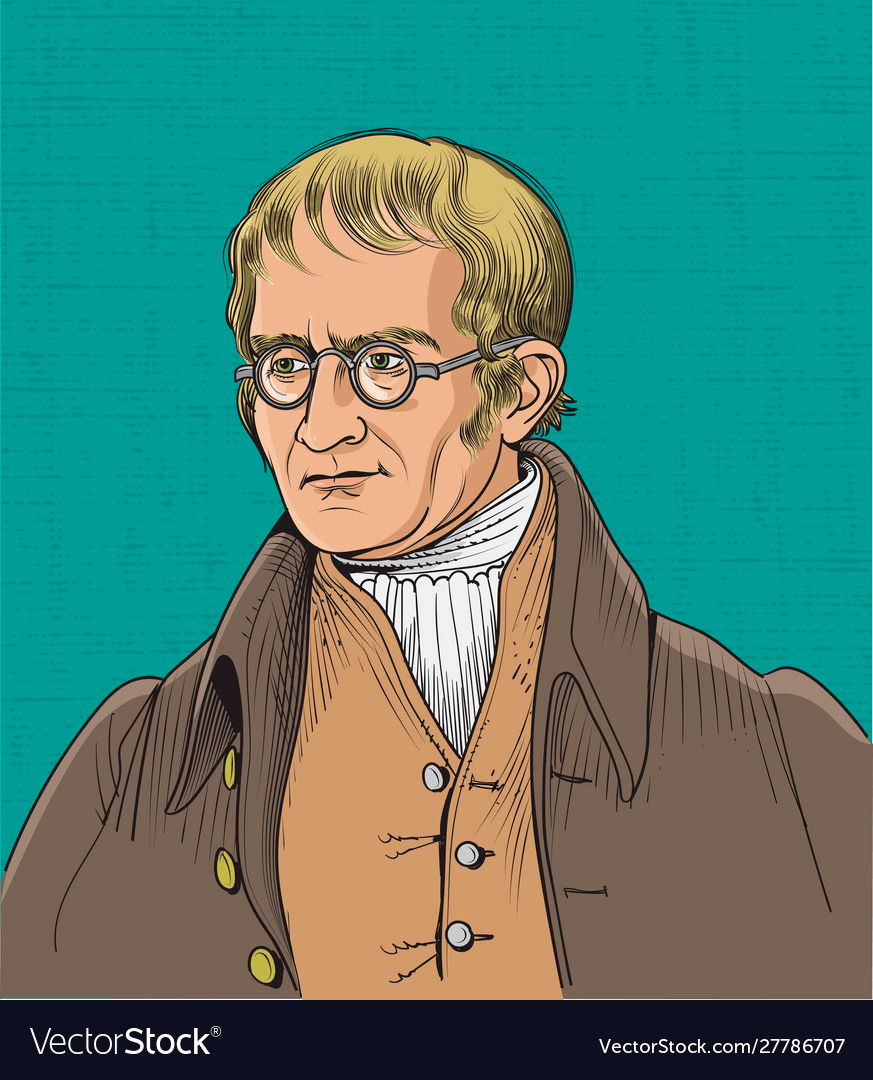 A English chemist who had concluded that atoms are the building blocks of elements. Atoms of one element are the same and different atoms can combine to form compounds.
A English chemist who had concluded that atoms are the building blocks of elements. Atoms of one element are the same and different atoms can combine to form compounds.
John Dalton
The element Na a.k.a Sodium has an atomic number of 11. Where do the electrons go according to their rings?
1st Ring:
2nd Ring:
3rd Ring:
1st Ring : 2
2nd Ring : 8
3rd Ring : 1
Where do you find the outermost layer, and highest energy? _________________ electrons
Valence Electrons
The forces of attraction between + and - ions. They are formed when atoms lend or borrow electrons.
Ionic Bonds
Baking Soda is an example of an
Base
 A chemist credited with the discovery of the electron. He received the Nobel Prize in physics in 1906 for this discovery.
A chemist credited with the discovery of the electron. He received the Nobel Prize in physics in 1906 for this discovery.
J.J Thomson
Label the Element Tile:
Atomic Number:
Chemical Symbol:
Element Name:
Atomic Mass:
Mass Number (Rounded):
Protons:
Neutrons:
Electrons:

Atomic Number: 22
Chemical Symbol : Ti
Element Name : Titanium
Atomic Mass: 47.867
Mass Number Rounded: 48
Protons : 22
Neutrons : 47.867(48) -22= 26
Electrons: 22
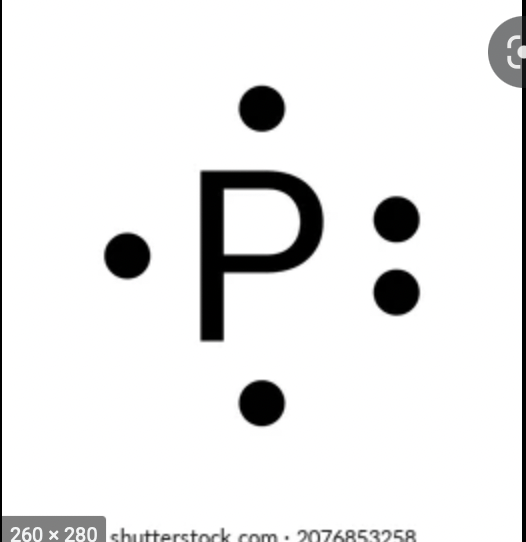
electron dot diagram for for phosphorus
Between nonmetals only! In a _________ bond, atoms share valence electrons.
Covalent Bonds
Lemon is an example of an
Acid
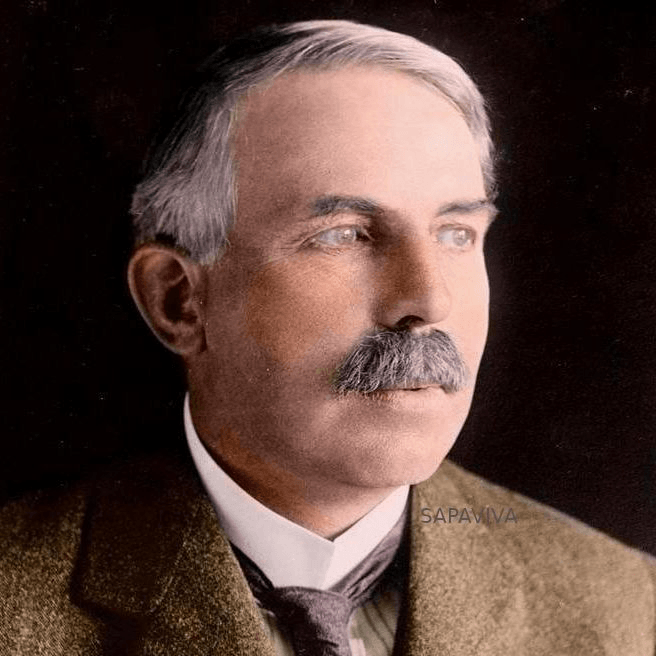 A former student of Thomson used a Gold Foil Experiment that showed a positive charge at the center of an atom called a proton.
A former student of Thomson used a Gold Foil Experiment that showed a positive charge at the center of an atom called a proton.
Ernest Rutherford
Label the Element Tile:
Atomic Number:
Chemical Symbol:
Element Name:
Atomic Mass:
Mass Number (Rounded):
Protons:
Neutrons:
Electrons:

Atomic Number:
Chemical Symbol:
Element Name:
Atomic Mass: 10
Mass Number (Rounded): 20.180
Protons: 10
Neutrons:20-10=10
Electrons:10
The first energy(electron) shell is located where?
Closest to the nucleus
Substances form when atoms from TWO or more elements band together
Compound
Acid comes from Latin word Acids which means?
Sour tasting or corrosive
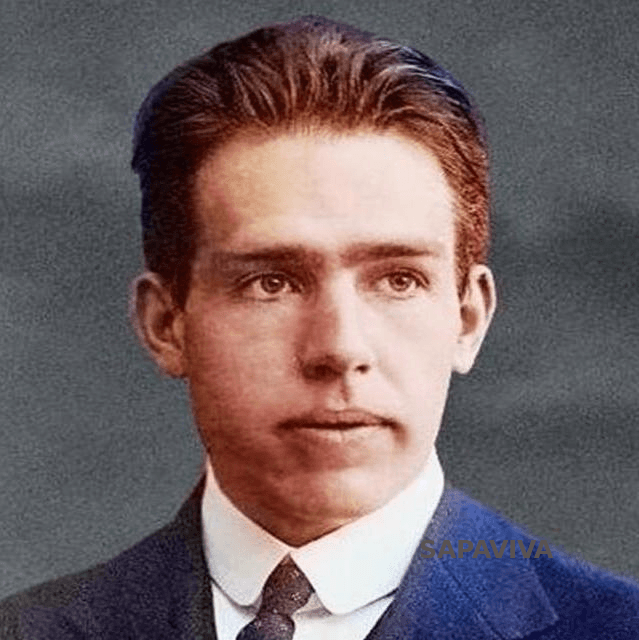 A Danish scientist who worked with Rutherford, and described the motion of electrons around the nucleus which lead to the model we use today to show electrons and an atom.
A Danish scientist who worked with Rutherford, and described the motion of electrons around the nucleus which lead to the model we use today to show electrons and an atom.
Niels Bohr
This elements are the most reactive of all metals; and don't occur in nature in their element form. What are the elements in Group 1 called?
Alkali Metals
The Periodic Table is organized their __________.
Atomic Number
A substance that indicates or shows if something is an acid or a base by changing color
Indicator
What technological advancement in 1590 changed the way we looked at Atomic Theory forever?
Microscope
These elements in groups 3-12 are the most familiar metals because they often occur in nature uncombined. They take you from the left side of the table to the right side of the table.
Transition Metal Elements
Did JJ Thomson win a Nobel Prize for his work? True or False
True
Anything above 7 on the PH Scale is called?
Base
What is the smallest particle that still can be considered an element?
Atom
What is the Periodic Table organized by?
Groups & Periods
Who organized the first periodic table?
Dmitri Mendeleev
How many electrons go in the Electron dot diagram of Oxygen?
6 electrons dots
What is 7 on the PH Scale?
Ex. Water or Blood
What does the word Atoms mean in Greek?
"Uncuttable"
What is the name of group 17 on the periodic table?
Halogens
Who improved the Periodic Table to what we know today?
Henry G.J Moseley
A material is one that can be pulled out, or drawn, into long wires.
Ductile
What is the scale called to measure Acid and Bases?
PH Scale
What would you call matter composed of ONE TYPE of atom?
Element
Create Atomic Model for phosphorus
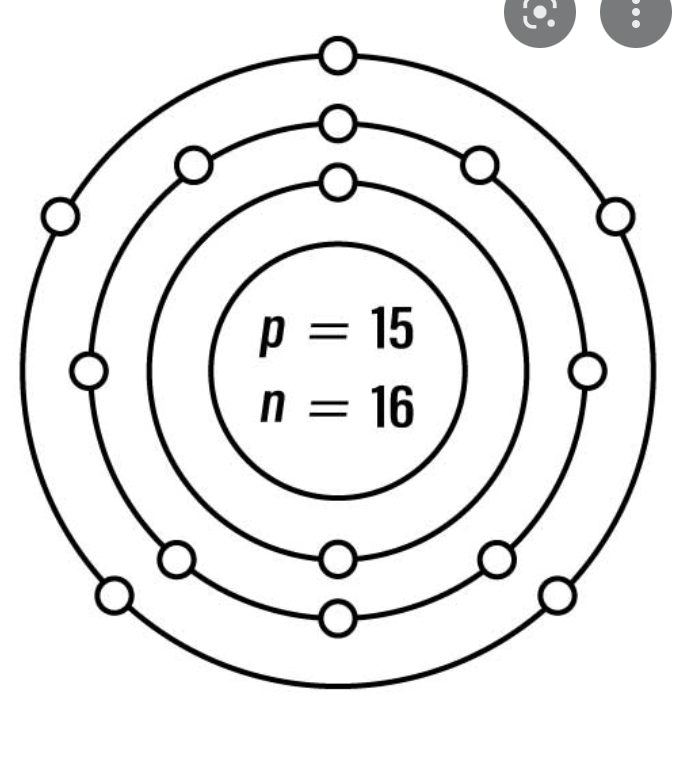
 How many Neutrons in Silver?
How many Neutrons in Silver?
A material that is shiny and reflective.
Luster
Which two items are the most acidic ?
Stomach and Car Battery
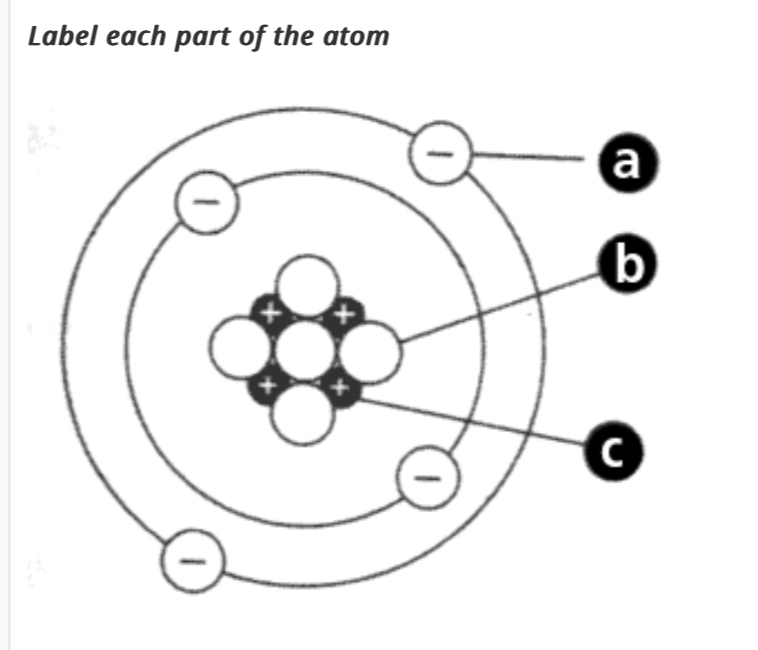
A. Electron
B. Neutron
C. Proton
Extra Credit: Name the groups in order 1 to 18. (5 points)
1:
2:
3-12:
13:
14:
15:
16:
17:
18:
1. Akali Metals
2. Akaline Metals
3-12: Transition Metals
13. Boron
14. Carbon
15. Nitrogen
16. Oxygen
17. Halogen
18. Noble Gases
 How many Neutrons in Cobalt?
How many Neutrons in Cobalt?
59-27=32
A material is one that can be hammered or rolled into flat sheets or other shapes.
Malleable
An indicator that is commonly found in the lab. This can be RED or BLUE
Litmus Test