A single kind of matter that always has a specific makeup or composition.
Example: Sea Salt
What is Substance?
The basic particle from which ALL elements are made. It is the SMALLEST particle of matter that makes up an element.
What is an ATOM?
This mixture has substances that are so EVENLY mixed that you can't see them and they can easily be separated.
What is Homogeneous Mixture?

This property describes how compact the matter is in a substance.
What is Density?
This change alters form or appearance of matter but DOES NOT turn any substance in the matter into a different substance.
When an object begins to rust because of Reactivity with Oxygen, Chemical or Physical Property?
Example: Statue of Liberty Rusting , Car rust, Metal rust.
What is Chemical Property?
A single kind of matter that is pure, meaning it is made of only ONE type of atom or molecule.
Example: Table Salt or an Element
What is PURE Substance?
A group of 2 or more atoms held together by chemical bonds.
What is Molecule?
This mixture is made up of particles that you can usually see and they can easily be separated.
What is a Hetergeneous Mixture?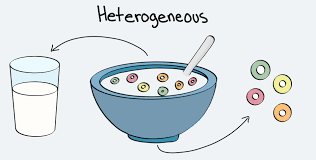
This property describes how light reflects off a substance.
What is Color?
This change in matter produces one or more NEW SUBSTANCES.
What is Chemical Change?
The Chopping of Wood in this scene is an example of what type of change?
What is Physical Change?
Anything that takes up space which has mass and volume.
What is Matter?
Density Formula
What is Density=Mass/Volume?
This mixture is a single kind of matter that contains a SOLVENT and a SOLUTE.
What is a Solution?
This property describes how shiny a substance is.
What is Luster?
In our Smores Lab, the chewing of food, melting of chocolate, and breaking a graham cracker was all what type of change?
What is Physical Change?
Baking a cake, rotting banana,fireworks, and burning wood are all examples of what type of change?
What is Chemical Change?
The measure of how much matter is in an object.
What is Mass?
A PURE substance that cannot be broken down into any other substance by chemical or physical means.
Examples:
Copper is Copper
Oxygen is Oxygen
What is Element?
A mixture containing small,undissolved particles that do not settle out. The particles are too small to be seen without a microscope, yet large enough to scatter a beam of light.
Example: Smoke
What is Colloid?
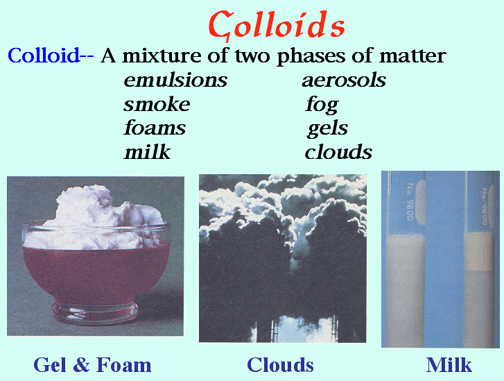
This property describes the smell of a substance.
What is Odor?
 This is an example of what type of Mixture?
This is an example of what type of Mixture?
B. Colloid
C. Suspension
What is Solution?

What is the Density of this gauntlet?
Density= Mass/Volume
D=1,000/100= 10g/cm3
The amount of space taken up by an object.
What is Volume?
2 or more substances that are together in the same place, but their atoms are NOT CHEMICALLY BONDED.
What is Mixture?
A mixture in which particles can be easily separated by settling or filtration. These are mixtures that don't mix.
Example: Muddy Water
What is Suspension?
This property describes a substances ability to RESIST shape change.

What is Hardness?

This is an example of what type of Mixture?
A. Solution B. Colloid C. Suspension
What is Colloid?

The McLaren P1 came out with a matchbox version of the car that has a mass of 6 grams and a volume of 12 cm3. What is the Density of the matchbox car?
D= 6g/12cm3
D=0.5 g/cm3