The smallest piece of an element.
What is an atom?
The 5 signs of a chemical change, name 2 of them
1. Color Change
2. Gas Production
3. Production of a Precipitate (Chunky Solid)
4. Temperature Change (internally)
5. Energy Change (heat, light, sound production)
In the following reaction, how many atoms of each kind are present on either side of the equation:
CH₄ + 2O₂ → CO₂ + 2H₂O
1 Carbon atom
4 Hydrogen atoms
4 Oxygen atoms
Energy of heat
Thermal Energy

At what point on the track does the car have the greatest potential energy?
The Top of the first and highest hill
An arrangement of atoms chemically combined to make a new substance.
What is a molecule?
You can always tell a chemical change occured if . . .
a new substance if formed
In the following reaction, how many atoms of each kind are present on either side of the equation:
N₂ + 3H₂ → 2NH₃
2 nitrogen atoms
6 hydrogen atoms
Explain the difference between endothermic and exothermic reactions
Endothermic Reactions absorb heat
Exothermic Reactions release heat

At what point on the track does the car have the greatest kinetic energy?
At the bottom of the first and highest hill, when it is closest to the ground
A positively charged particle in the nucleus of an atom
What is a proton?
Give two positives of a synthetic polymers?
Strength
flexibility
easy to clean
durability
In the following reaction, how many atoms of each kind are present on either side of the equation:
2 H₂ + O₂ → 2 H₂O
2 oxygen atoms
4 Hydrogen atoms
The formula for Kinetic Energy
KE = 1/2 x Mass x speed2

What is the kinetic energy of the ride turned into as the ride goes on. Include at least 2 energy transformations.
Electric, Sound, Thermal
A negatively charged particle that orbits the nucleus of an atom
What is an electron?
Give two negatives for synthetic polymers . . .
Pollution
does not break down
difficult to dispose of
microplastics causing health concerns
Based on this molecule diagram, what is the chemical symbol for Sulfuric Acid.
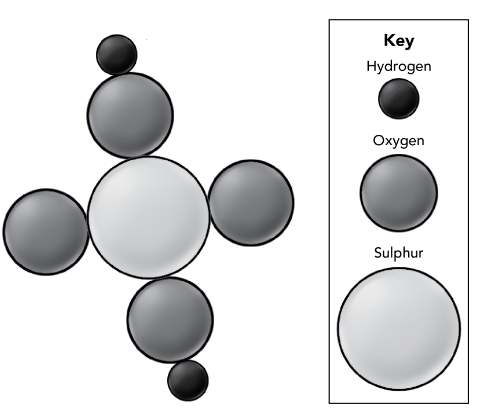
H2SO4
Formula for gravitational potential energy
GPE = Weight x Height
Sunlight enables plants to use water and carbon dioxide during photosynthesis to make and store sugar. Any animal that eats the plants can convert the sugar into the energy it needs to move from place to place. Which three types of energy are involved in this example?
chemical, thermal, electromagnetic (light), kinetic
A neutrally charged particle in the nucleus of an atom
What is a neutron?
What is Law of Conservation of Mass?
No atoms are created or destroyed in a chemical reaction. They are only reorganized.
Crushing Aluminum cans would be a ________ change (physical or chemical)
Physical
Name 2 types of energy and where you would find them in everyday life.
Nuclear Energy
Chemical Energy
Thermal Energy
Electrical Energy
Kinetic
Potential
Where you find them (Answers may vary.)
Give an example of energy transformations
Toaster = Electrical > Thermal
Baking Bread = Thermal > Chemical
Power Plant = Kinetic > Electrical
Digesting Food for Work = Chemical > Kinetic
Burning Firewood = Chemical > Thermal
Fan = Electrical > Kinetic
Nuclear Power Plant = Nuclear > Electrical
Battery = Chemical > Electrical
Falling = Potential > Kinetic
An interaction between two substances where a new substance if formed.
What is a chemical reaction/chemical change?
In the following reaction, the subscript (little number) tells how many _________ per __________
N₂ + 3H₂ → 2NH₃
atoms per molecule
The coefficient (big number) tells how many ____________
The subscript (little number) tells how many ____________
molecules
atoms
Name the two types of potential energy beside gravitational
Elastic and Magnetic
Give an example of energy transformation
Toaster = Electrical > Thermal
Baking Bread = Thermal > Chemical
Power Plant = Kinetic > Electrical
Digesting Food for Work = Chemical > Kinetic
Burning Firewood = Chemical > Thermal
Fan = Electrical > Kinetic
Nuclear Power Plant = Nuclear > Electrical
Battery = Chemical > Electrical
Falling = Potential > Kinetic