Which of the following statements correctly compares the atomic structures of solids and gases?
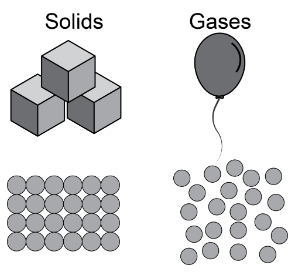
A. Particles in solids are loosely packed and can move around, while particles in gases are arranged in a tight, repeating pattern.
B. Particles in solids are arranged in a tight, repeating pattern, while particles in gases are loosely packed and can move around.
C. Particles in solids can move around more easily than those in a gas.
D. Particles in gases are packed more tightly than those in a solid.
B. Particles in solids are arranged in a tight, repeating pattern, while particles in gases are loosely packed and can move around.
When panning for gold, a miner scoops up gravel, rocks, and dirt, and then washes the mixture with water, leaving the gold behind. What is one reason the gold is easily separated from the gravel, rocks, and dirt?
A. The gold is easily separated because the mixture is a pure substance. This means it is easily separated by physical means.
B. The gold is easily separated because the mixture is a homogeneous mixture. This means it is easily separated by physical means.
C. The gold is easily separated because the mixture is a heterogeneous mixture. This means it is easily separated by physical means.
D. The gold is easily separated because the mixture is a solution. This means it is easily separated by physical means.
C. The gold is easily separated because the mixture is a heterogeneous mixture. This means it is easily separated by physical means.
- Breaks apart easily when hit with a hammer
- Dull appearance
- Low melting point
- Unable to conduct electricity
- A. The students tested a metalloid because the physical properties are of both a metal and nonmetal.
- B. The students tested a metal because the physical properties describe metals.
- C. The students tested a nonmetal because the physical properties describe nonmetals.
D. The students need to conduct additional investigations to determine more physical properties before the unknown element can be classified.
C. The students tested a nonmetal because the physical properties describe nonmetals.
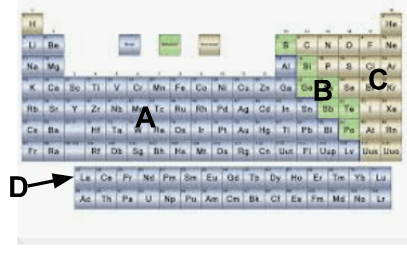
What group of elements is labeled A?
A. Metals
What is this called?
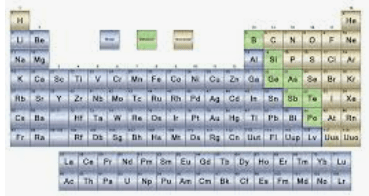
The Periodic Table of Elements
The kinetic energy of atoms in solids–
A. is lower than liquids but higher than gases.
B. is higher than liquids but lower than gases.
C. does not exist. Only liquids and gases have kinetic energy.
D. is much lower than the energy in liquids and gases.
D.is much lower than the energy in liquids and gases.
Which of the following is NOT an example of a homogeneous mixture (solution)?
A. Sodium and chlorine chemically combine to create salt
B. Water and lemonade powder are mixed to create lemonade.
C. Salt is dissolved in water to create salt water.
D. Milk is poured into coffee and mixed.
A. Sodium and chlorine chemically combine to create salt
The Periodic Table can be broken into three different sections. In which section would you expect to find an element that is a semiconductor, shiny, and brittle?
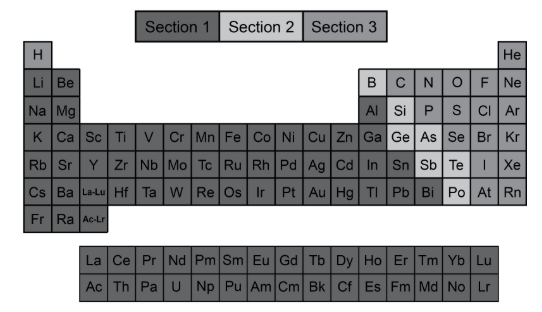
A. An element that is a semiconductor, shiny, and brittle would be a metal; therefore, it can be found in Section 1.
B. An element that is a semiconductor, shiny, and brittle would be a metal; therefore, it can be found in Section 3.
C. An element that is a semiconductor, shiny, and brittle would be a metalloid; therefore, it can be found in Section 2.
D. An element that is a semiconductor, shiny, and brittle would be a metalloid; therefore, it can be found in Section 3.
D. An element that is a semiconductor, shiny, and brittle would be a metalloid; therefore, it can be found in Section 2.
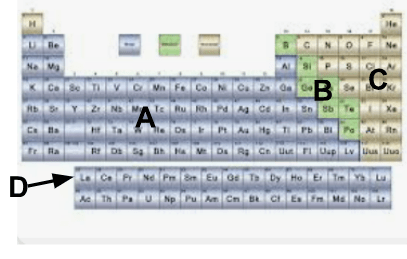
What group of elements is labeled B?
B. Metalloids
What state of matter is this image showing?
Gas
What is happening to the molecules as the flame heats the water? 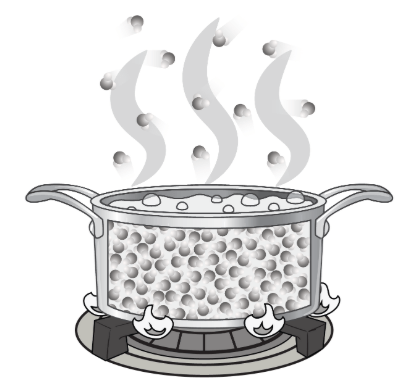
A. As heat energy is taken away, the volume of the water becomes more definite.
B. The heat energy causes the molecules to move more slowly and clump together, taking up less space.
C. Heat energy causes the molecules to move faster, so they are farther apart and take up more space.
D. The molecules grow larger and take up more space, so the volume increases.
C. Heat energy causes the molecules to move faster, so they are farther apart and take up more space.
What type of mixture is being described?
-Can easily be separated using physical means
-Has a uniform appearance
A. Homogeneous mixture
B. Heterogeneous mixture
C. Pure Substance
A. Homogeneous mixture
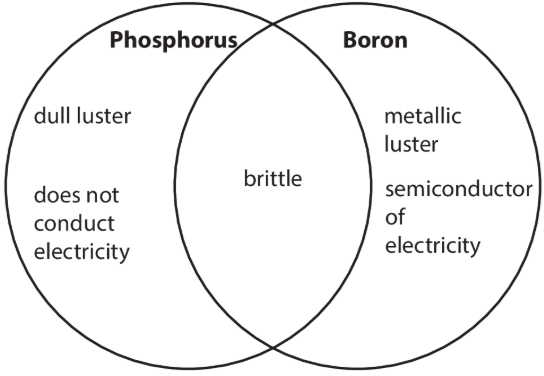
Based on the diagram, which of the following is a claim the student can make?
A. Boron is a nonmetal.
B. Phosphorus is a metalloid.
C. Boron is a metalloid.
D. Phosphorus is a metal.
C. Boron is a metalloid.
How many known elements are there on the periodic table?
118 known elements
Mixture or Pure substance?

Pure Substance
A. Liquids take the shape of their containers, while gases spread out and fill the entire container.
B. Liquids never take the shapes of their containers, while gases usually do.
C. Particles in a liquid form a straight line, and particles of a gas do not.
D. The particles in a liquid have a definite shape, while those in gases do not.
A. Liquids take the shape of their containers, while gases spread out and fill the entire container.
Which of the following is NOT a method used to determine if a substance is a mixture or a pure substance?
A. Chemical separation
B. Evaporation
C. Straining with a sieve
D. Tweezers
A. Chemical separation
Yttrium (Y) is silver, metallic, soft and ductile when mixed with other metals, never found in large amounts, and found mixed with other elements. It has been used in creating high temperature superconductors. Based on these properties, yttrium would be classified as a –
A. Nonmetal
B. Metalloid
C. Metal
D. Rare Earth element
D. Rare Earth element
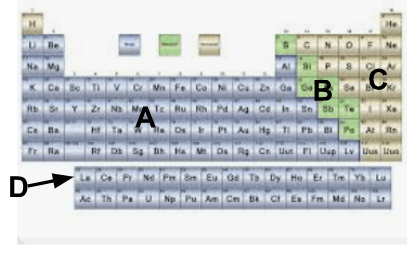
What group of elements is labeled C?
C. Nonmetals
Heterogeneous or Homogeneous?

Heterogeneous
What happens to the kinetic energy of the atoms in a liquid as it is cooled, and how does their movement change?
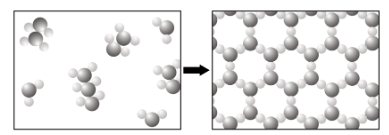
A. The atoms move slower, and the liquid becomes a gas.
B. The atoms move faster, spread out, and become a gas.
C. The atoms begin moving faster, and the substance becomes a solid.
D. The atoms move slower, and they take on a more regular structure.
D. The atoms move slower, and they take on a more regular structure.
Can easily be separated using physical means.
Does not have a uniform appearance.
A. Homogeneous mixture
B. Heterogeneous mixture
C. Pure Substance
B. Heterogeneous mixture
The table shows the classification and physical state of four elements. Which element most likely has a shiny luster?
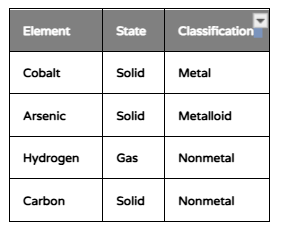
A. Cobalt
B. Arsenic
C. Hydrogen
D. Carbon
A. Cobalt
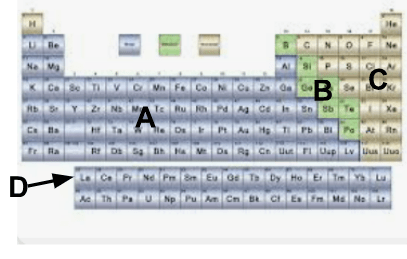
What group of elements is known as D?
D. Rare earth elements
Heterogeneous or Homogeneous?

Homogeneous