What is the name of the outermost electrons?
Valence electrons
What are the 5 kinds of reactions you typically see in class/textbook?
Combination, Decomposition, Single Replacement, Double Replacement, Combustion
Using
∆E = (-2.18 x 10-18 J) ((1/nf2) - (1/ni2)),
how much energy was released when an electron jumps to n=1 from n=3?
-1.94 x 10-18 J
What hybridization does this molecule have?
H - N - H
|
H
sp3
What is the formal charge for each atom in the molecule:
CCl2O
C: 0
Cl: 0 for both
O: 0
What are the names of the Group 7 and 8 elements?
Halogens and Noble Gases
What two anions are always soluble?
NO3- and CH3COO-
What is the trend of Z(effective) across a period? Explain
Across a period, Z(eff) increases because the number of protons increases while the number of shielding electrons (Core electrons) remains the same
In _________ bond(s), bonding electrons are shared such that all particles, after bonding, have the same number of electrons as a noble gas.
a) Nonpolar covalent bonds
b) Polar covalent bonds
c) Ionic bonds
d) A and B
e) A, B, and C
d) A and B
How many grams of K2SO4 are needed to react with 85.3 g of AlBr3?
83.6 g K2SO4
Which subatomic particles are the same in a given set of isotopes?
Protons and Electrons
Calculate the enthalpy for this reaction:
2C(s) + H2(g) ----> C2H2(g)
Given the following equations:
C2H2(g) + 5/2 O2(g) ---> 2CO2(g) + H2O(l) ΔH°= -1299.5 kJ
C(s) + O2(g) ---> CO2(g) ΔH° = -393.5 kJ
H2(g) + 1/2 O2(g) ---> H2O(l) ΔH° = -285.8 kJ
+226.7 kJ
Which term states that when filling orbitals in a subshell, each one should have one electron before adding another with the opposite spin?
Aufbau Principle
Hund's Rule
Pauli-Exclusion Principle
Hund's Rule
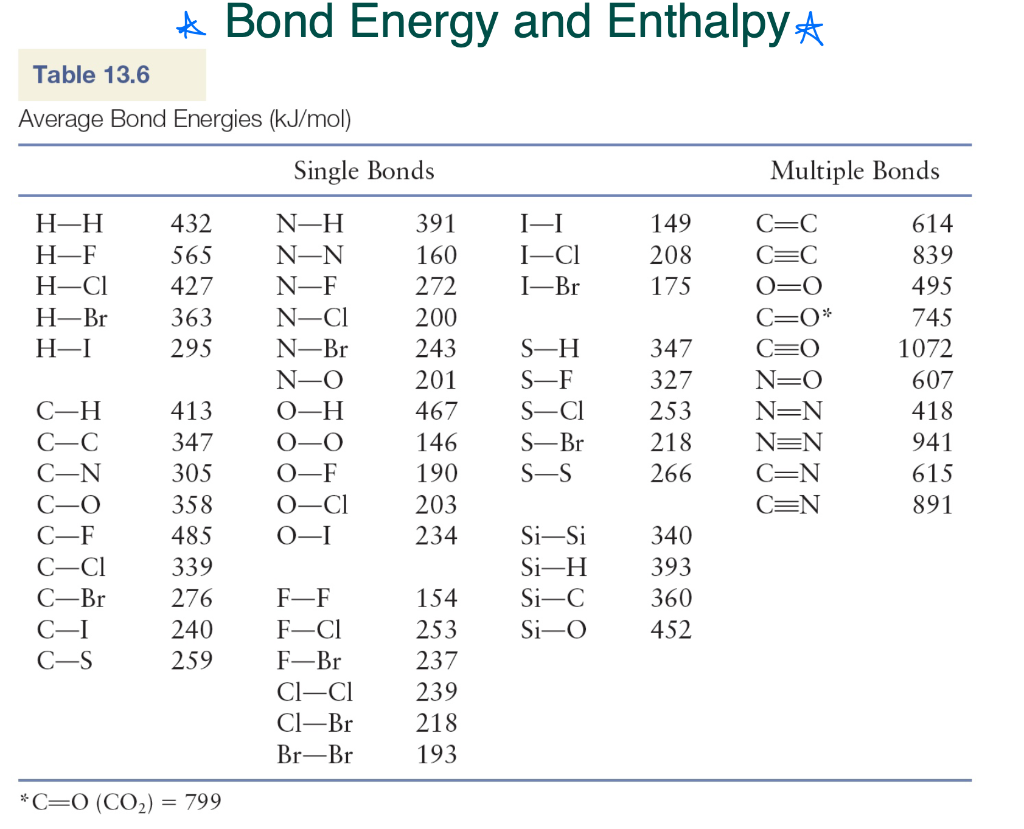
Find the bond enthalpy of the unbalanced reaction (make sure to balance):
C3H8 + O2 ----> CO2 + H2O
-2060 kJ/mol
A balloon inflated with three breaths of air has a volume of 1.92 L. At the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
5.12 L
In the combustion of 67.32g of butane (C4H6), how many grams of CO2 are produced?
Write and balance the equation before solving
203.8 g CO2
A 23.6 g sample of Ethanol releases 4151 J as it cools from 95.8 oC. Calculate the final temperature of the ethanol.
Use 2.46 J/g C for specific heat of Ethanol
24.3 oC
What is the electron configuration of Cr+3?
1s22s22p63s23d3
What is the electron (domain) geometry, molecular geometry, and hybridization of the central atom for the ICl4- ion?
Electron geometry: Octahedral
Molecular geometry: Square Planar
Hybridization: sp3d2
Find the heat of formation for a mole of carbon monoxide given:
CO2(g) + C(s) ----> 2CO(g) ∆H = 172.5 kJ
C(s) + O2(g) ----> CO2(g) ∆H = -393.5 kJ
Use kJ and 3 sig figs in your final answer
C(s) + 1/2 O2(g) ----> CO(g)
∆Hof = -1.10 x 102 kJ
Balance the equation:
S + HNO3 ----> H2SO4 + NO2 + H2O
S + 6 HNO3 ----> H2SO4 + 6 NO2 + 2 H2O
What is the concentration of Sr(OH)2 if a 2 L solution of containing 45.3 g of HCl was used, and only 65.9 mL of HCl was needed to neutralize 12.4 mL of Sr(OH)2?
1.65 Molar Sr(OH)2
What is the energy of a photon with a wavelength of 325 nm? What about the energy of one mole of these photons?
First answer should have the unit J/photon
Second answer should have the unit kJ/mol
6.11 x 10-19 J/photon
368 kJ/mol
Draw the lewis dot structure for H2SO4 (H is bonded to O)

If a square piece of gold foil contains about 1.73 x 1022 atoms of Au and the thickness is 0.0000173 cm, what is the length of one side of the foil?
Density of gold: 19.3 g/mL
1.30 x 102 cm