Molecular geometry of CO2?
linear
what's the hybridization of the carbon in HCN?
sp
what intermolecular force is found in all molecules?
london dipsersion force
The mass percent of C in acetone (C3H6O)
62%
what is the formula for sodium phosphate
Na3PO4
An example of a tetrahedral electron geometry
(any molecule with 4 electron groups)
is B2 paramagnetic or diamagnetic? (draw orbital diagram)
paramagnetic
what are the 3 elements that can do hydrogen bonding?
F, O, and N
balance the equation LiClO -> Li2 + Cl + O4
4LiClO -> 2Li2 + 4Cl + O4
true or false?
intermolecular forces are within molecules, while bonds are between molecules
false!
which of the following does NOT have t-shaped molecular geometry?
BrF3, ClF3, NH3
NH3
what is the hybridization of xenon dichloride?
sp3d
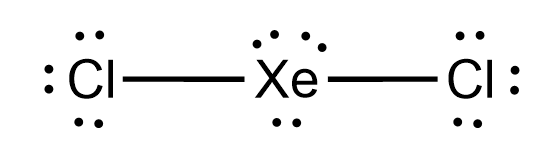
what forces are present in IF5?
how many grams of aluminum will i need in order to make 3.2 grams of hydrogen gas?
2Al + 6HCl -> 2AlCl3 + 3H2
28.55 grams aluminum
why are ion-dipole forces stronger than hydrogen bonding?
(anything about electronegativity)
The molecular and electron geometry of XeCl4
square planar (for molecular) and octahedral (for electron)
true or false?
the pi component of a double bond is stronger than the sigma component
false!
true or false?
CH2F2, seesaw shaped molecules, and CH3COOH are all polar molecules
true!
when 1.41 grams of ammonia react in the following equation, how many grams of nitrogen gas are produced?
4NH3 + 3O2 -> 2N2 + 6H2O
1.16 grams
in a triple bond, how many sigma and pi bonds are there?
1 sigma and 2 pi
rank the following in order of increasing (smallest to biggest) bond angle
tetrahedral, octahedral, bent
octahedral < tetrahedral < bent
between F2 and F2-, which would have a shorter bond? (hint: find bond orders)
F2
briefly explain why water (H2O) would have a higher boiling point than methane (CH4)
water has stronger intermolecular forces, boiling point increases with increasing forces, etc.
butane (C4H10) reacts with oxygen gas (O2) in a combustion reaction to form carbon dioxide and water vapor. what is the sum of the coefficients of the balanced equation?
33
2C4H10 + 13O2 -> 8CO2 + 10H2O
categorize each of the following as either a physical property or chemical change:
boiling point of ethanol, combustion of octane, thickness of maple syrup, rust forming on a nail
chemical: combustion, rust forming