Which phase has a definite shape and volume?
solid
When volume decreases, pressure ___________.
increases
A substance that is a good conductor
metal
Is density a size-dependent or size-independent property?
size-independent!
What is flammability?
the ability of a type of matter to burn easily
Which phase(s) are you able to easily compress?
gases
The transfer of thermal energy from an area of high temperature to low temperature
heat
A compound formed of atoms with opposite charges
ionic compound
Which of the following is a qualitative property?
- melting point
- mass
- density
- flammability
flammability
True or false: you can observe a chemical property without changing the substance
false!
Freezing goes from a _________ to a ____________.
liquid to a solid
According the the Volume-Temperature Law, if the pressure is constant, the volume of a gas will increase if the temperature ____________.
increases
What has a higher melting point: a nonmetal solid or a metal solid?
metal solid
If the mass of something is 100g and the volume is 50mL, what is the density?
INCLUDE UNITS👺👺👺👺👺👺👺👺👺
2g/mL
What is kinetic energy and which phase (solid, liquid, gas) has the LEAST amount of kinetic energy?
solids have the least (particles vibrate in place)
If a solid was brought to its melting point, then thermal energy was (added/removed) and the solid will go to a phase change to become (liquid/gas).
added, liquid
What type of covalent compound will dissolve in water?
polar covalent compound
Explain the difference between mass and weight
mass: amount of matter in a substance
weight: the pull of gravity on matter, location dependent
2Mg + O2 --> 2MgO
Name the product(s) in the reaction
2MgO
Explain what happens to the kinetic, potential, and thermal energy through condensation?
all 3 decrease
When you draw the graph, put pressure as the dependent variable.
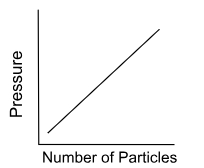 proportional
proportional
Of the following, which is not a characteristic of a metal?
- strong attraction between atoms
- malleable
- high kinetic energy
- high melting point
high kinetic energy
- Metals are extremely densely packed due to the attraction between atoms, therefore there isn't a lot of movement possible for the atoms
Name a property that you can use to identify matter
melting point, density
Explain the difference between the 2 in front of Li and the 2 after the F.
2Li : there are two separate molecules made of a single Lithium atom
F2 : there is a single molecule made of two fluorine atoms