Use the periodic table to determine the atomic number of the following element:
Helium (He)
2
Protons:32
Neutrons:42
Electrons:32
Name me. Use hyphen form
Germanium-74
63
Cu+
29
How many protons, neutrons, and electrons?
29, 34, 28
How many electrons are in each energy level of a neutral sodium atom?
Energy Level 1:
Energy Level 2:
Energy Level 3:
2, 8, 1
Which element is the most reactive? (sodium (Na) and rubidium (Rb).)
Rb
Compare the atomic structure of iodine (I), fluorine (F) and tin (Sn)
Rank the Coulombic force of attraction between the valence electrons and the nucleus in each element from low to high.
Tin, Iodine, Fluorine
Measuring that a candle lost 12 grams of mass is this type of observation.
Quantitative
What element is this neutral atom?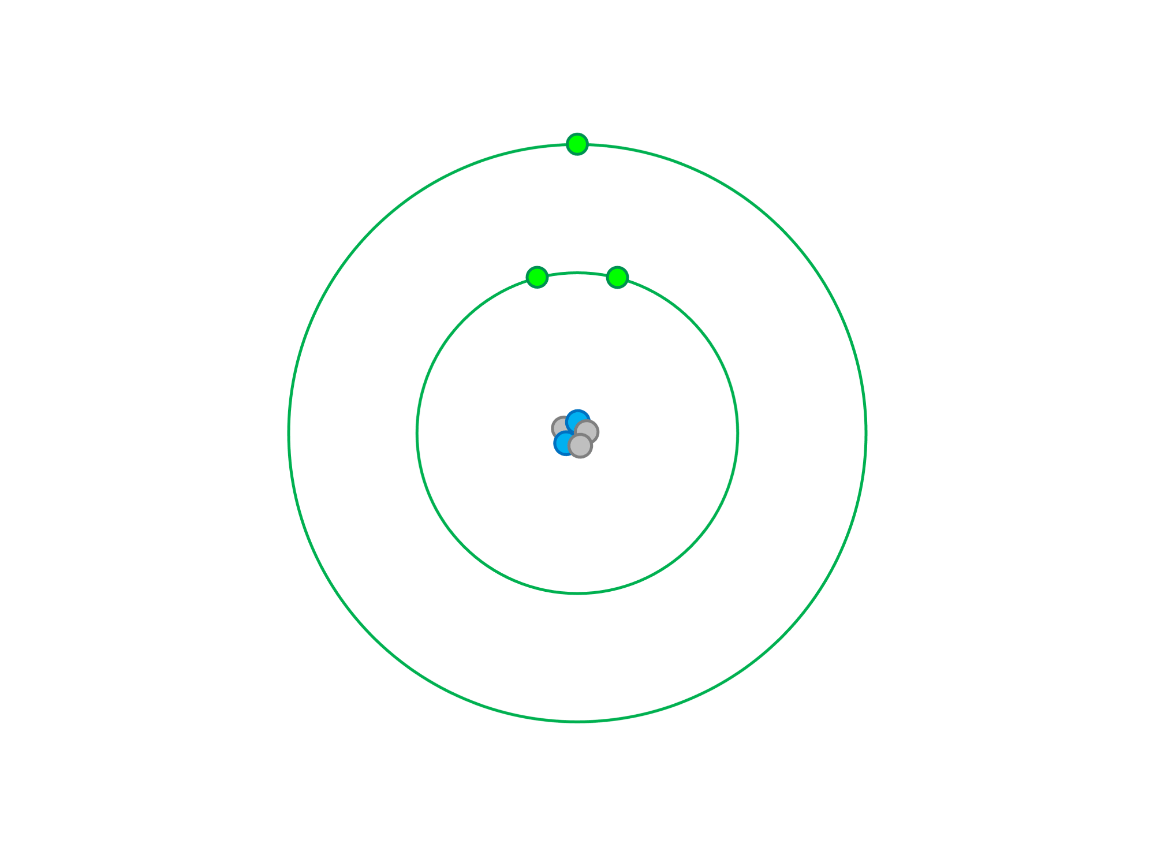
lithium
Use the periodic table to determine the atomic number of the following element:
Argon (Ar)
18
Protons:40
Neutrons:50
Electrons:40
Name me. Use hyphen form
Zirconium-90
44
Ca 2+
20
How many protons, neutrons and electrons?
20, 24, 18
How many electrons are in each energy level of a neutral phosphorus atom?
Energy Level 1:
Energy Level 2:
Energy Level 3:
2, 8, 5
Which element is the most reactive? (iodine (I) and chlorine (Cl))
Cl
Compare the atomic structure of phosphorus (P), antimony (Sb) and rubidium (Rb).
Rank the atomic radius of each element from low to high.
P, Sb, Rb
When wood burns in a campfire, this type of chemical reaction occurs, releasing heat, light, and new substances
Combustion
What element is this neutral atom?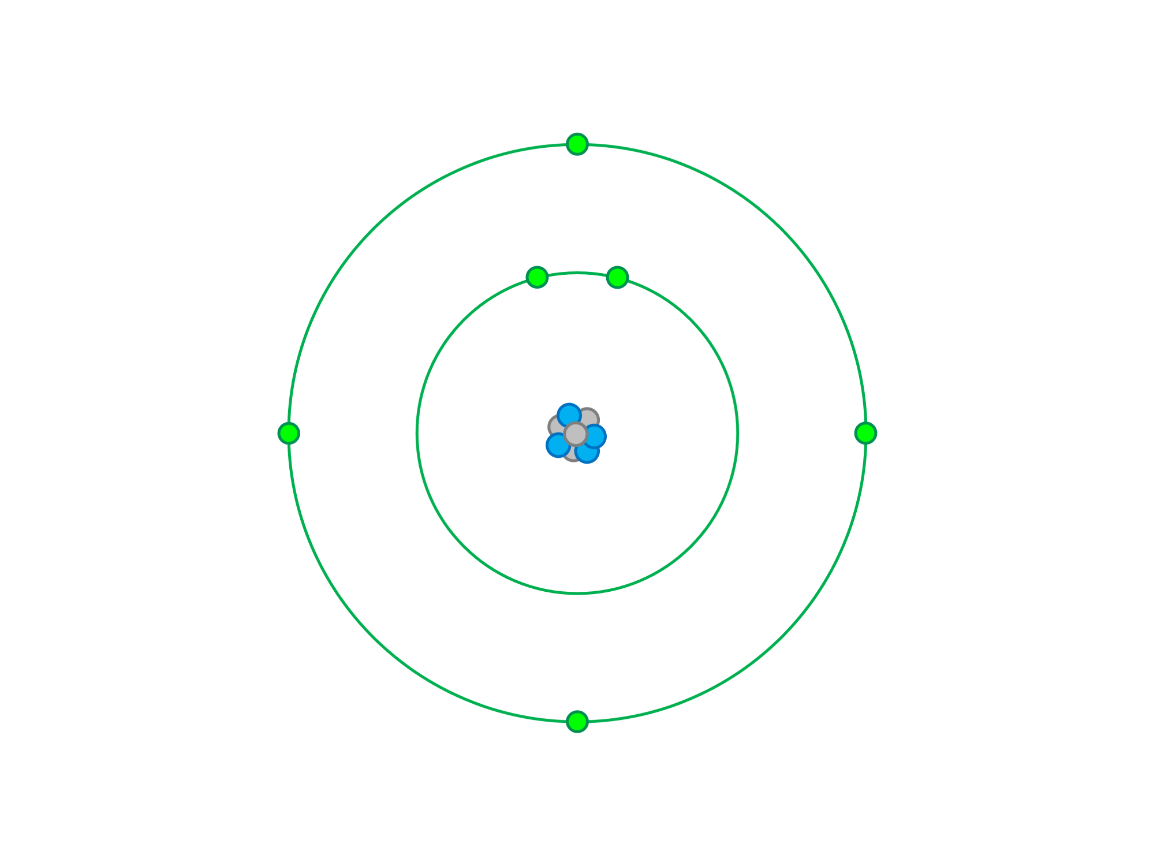
Carbon
Use the periodic table to determine the atomic number of the following element:
Strontium (Sr)
38
Copper-65.
How many protons, neutrons and electrons do I have?
29, 36, 29
14
N 3-
7
How many protons, neutrons, and electrons?
7, 7, 10
How many valence electrons does a neutral nitrogen atom have?
5
Element Atomic Radius (pm)
Lithium 167
Potassium 243
Which of the following is the best estimate for the atomic radius of sodium (Na)
8, 167, 190, 243, 637
190
Compare the atomic structure of nitrogen (N), strontium (Sr) and beryllium (Be).
Rank the Coulombic force of attraction between the valence electrons and the nucleus in each element from low to high.
Strontium, Beryllium, Nitrogen
Saying a solution looks “cloudy and blue” is this type of observation.
Qualitative
What element is this neutral atom?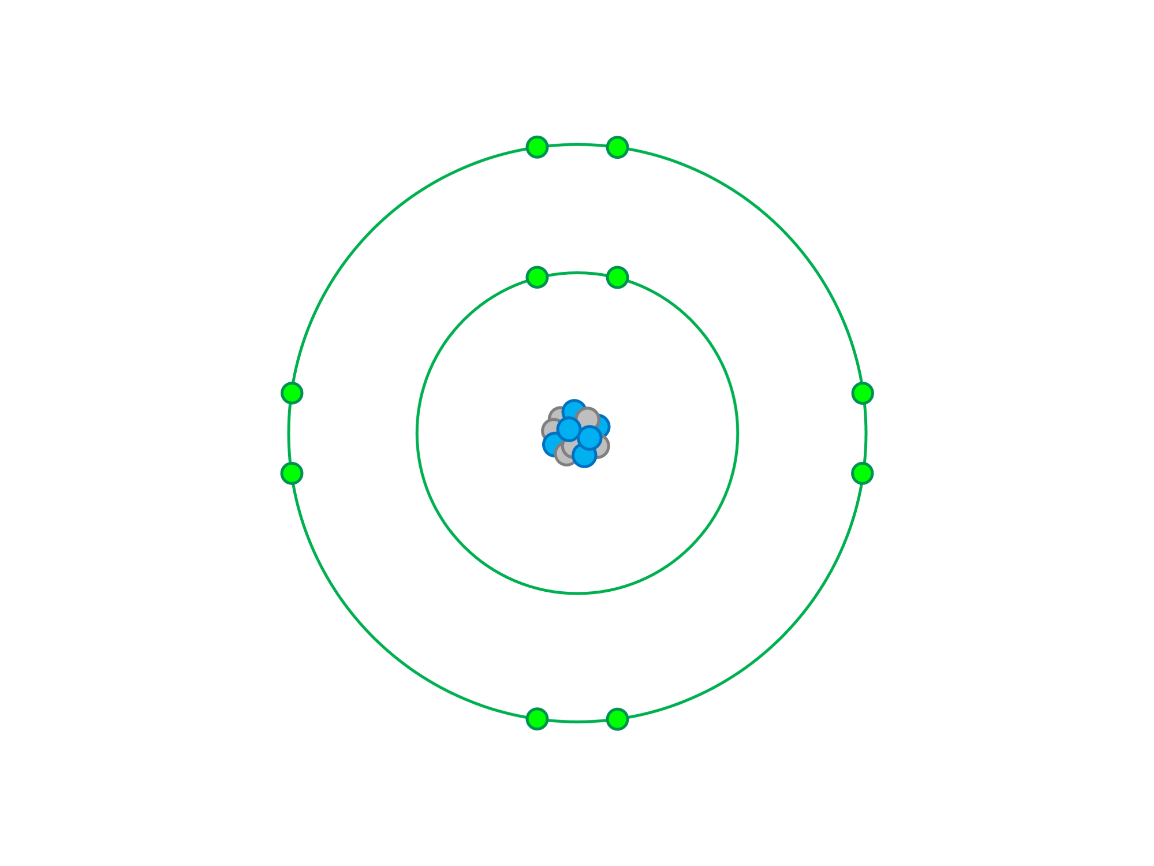
Neon
How many neutrons does Aluminum typically have?
14
I have 8 protons, and I'm feeling pretty good since I have 10 electrons. What's my name and charge?
Oxygen, 2-
What is different about Carbon-12 vs Carbon-13?
Number of neutrons
How many dots would be around a neutral Nitrogen Lewis Structure?
5
Element Electronegativity
Aluminum 1.61
Phosphorus 2.19
Which of the following is the best estimate for the electronegativity of silicon (Si)?
0.54, 1.61, 1.90, 2.19, 4.54
1.90
Compare the atomic structure of boron (B), indium (In) and fluorine (F).
Rank the metallic character of each element from low to high.
Fluorine, Boron, Indium
Liquid water boiling into a gas is a phase change or a chemical change?
Phase change
Which of the following ions could this Bohr diagram represent? F3- , Cl-, O2- , Be2+
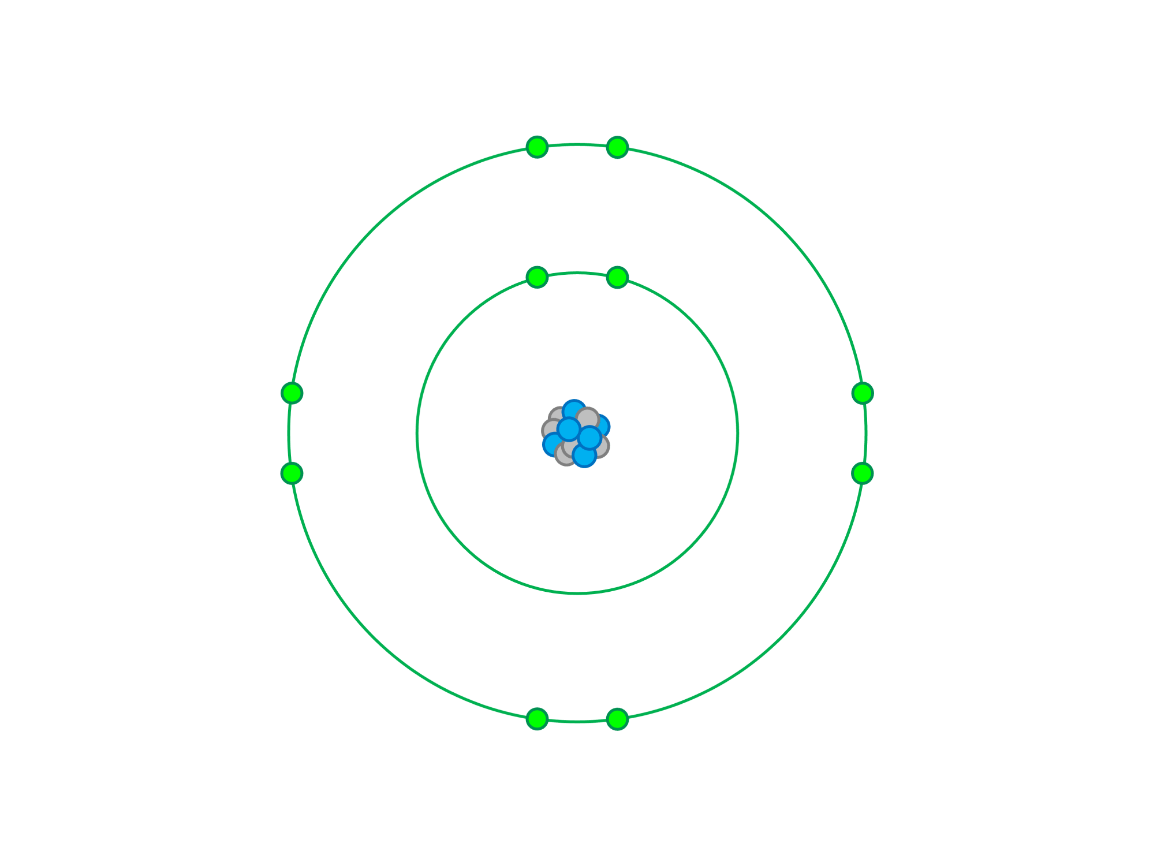
O2-
29
Si
14
How many protons, neutrons, and electrons?
14, 15, 14
My atomic mass is 40. I have 18 protons. How many neutrons do I have and what's my name?
22, Argon
How many neutrons does Carbon-14 have?
8
How many electrons are in each energy level of a sodium ion?
Energy Level 1:
Energy Level 2:
Energy Level 3:
2, 8, 0
Element Atomic Radius (pm)
Tellurium 123
Antimony 133
Which of the following is the best estimate for the atomic radius of iodine (I)?
115 pm, 123 pm, 128 pm, 133 pm, 397 pm
115
Compare the atomic structure of oxygen (O), tin (Sn) and carbon (C).
Rank the first ionization energy of each element from low to high.
Tin, carbon, oxygen
What are the reactants?
What are the products?
Reactants= gas and oxygen
Products= carbon dioxide and water
This is a Lewis dot diagram for a neutral atom in period 3. What element does this neutral atom belong to? 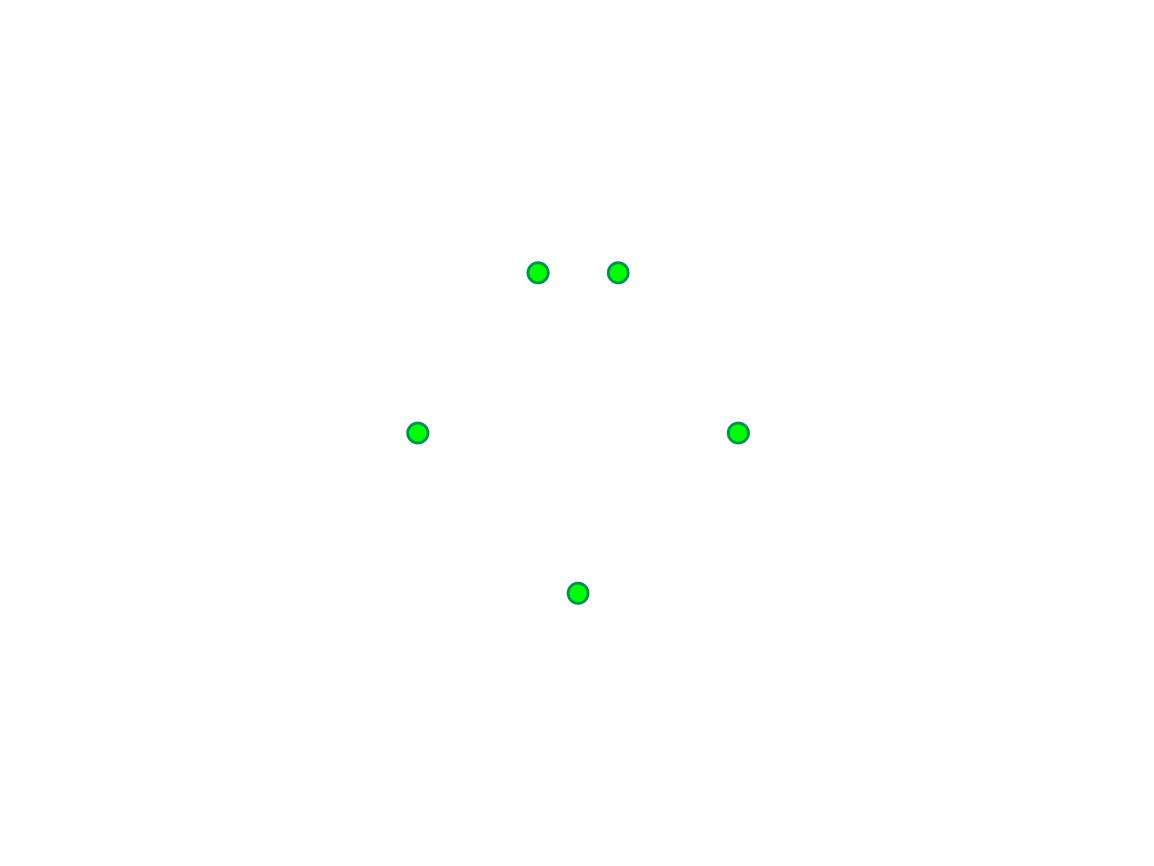
Phosphorous