What is the molar mass of H2SO4?
98.08 g/mol
The following image most likely represents an isotope of the element _____________. (x axis is in g/mol)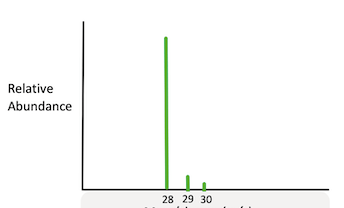
Silicon
A 2.4 g sample of a mixture of Calcium Chloride (CaCl2) and Sodium Chloride (NaCl), is found to contain 0.12 g of Na. What percent of this sample is NaCl?
13% NaCl
"The amount of energy required to remove an electron from an atom" is called what?
Ionization energy
Which atom below has a larger atomic radius, and why?
a. Calcium
b. Magnesium
c. Potassium
d. Sodium
How many atoms are in Al(NO3)3?
13
What is the average atomic mass of this isotope?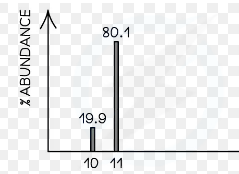
10.801 g/mol
Mixture or pure substance?
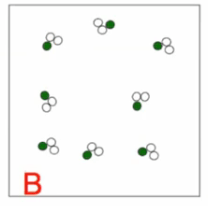
Pure substance
Looking at the diagram below, which electron (a, b, or c) would be easiest to remove? Justify your choice in terms of Coulomb's law.
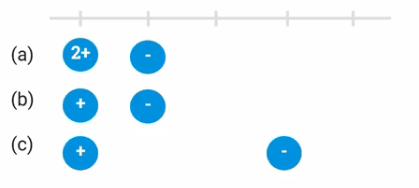
Electron C is easiest to remove. According to Coulomb's law, the force of attraction is proportional to the magnitude of the particle charges and inversely proportional to the distance between them.
Which of the following would most likely become an anion?
a. Kr
b. Br
c. C
d. B
b Br
A student obtains a sample of a pure solid compound. In addition to Avogadro's #, what two numerical values must the student obtain in order to calculate how many molecules are in the sample?
(1)The Molar mass of the compound and (2)the mass of the sample
What element is displayed in this PES Diagram? 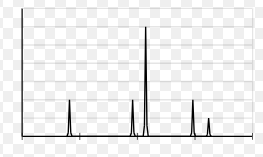
Aluminum
The mass percent of carbon in pure glucose (C6H12O6) is 40.0 percent. A chemist analyzes an impure sample of glucose and determines that the mass percent of carbon is 38.2 percent. Which of the following impurities could account for the low mass percent of carbon in the sample?
a. Water H2O
b. Ribose C5H10O5
c. Fructose C6H12O6 (an isomer of glucose)
d. Sucrose C12H22O11
Water
What is the electron configuration for Ca+2? Do not use noble gas formation.
1s2 2s2 2p6 3s2 3p6
Yes I agree. Atomic radius of F is smaller than Br. Coulomb's law states that force of attration is greater when distance is smaller. Since F is smaller than Br due to lack of shells, it will have a larger first ionization energy.
What percentage of the compound Al2O3 is Oxygen?
47%
How many electrons are in the outermost energy level of this atom? 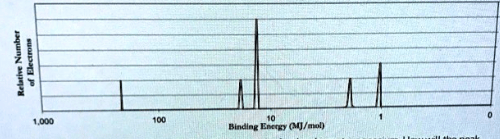
Cu + 4HNO3 --> Cu(NO3)2 + 2NO2 + 2H2O
A student analyzes a 2.00 g sample of a mixture of copper, Cu, and aluminum, Al, by reacting the copper with the nitric acid, HNO3, as shown in the equation above. The student determines that the reaction produces 0.010 mol Cu(NO3)2. Assuming that all of the copper in the mixture reacted completely, what was the percent of Cu by mass in the 2.00 g sample?
32%
Which of the following ground state electron configurations represents the atom that has the highest first ionization energy?
a. 1s2
b. 1s2 2s1
c. 1s2 2s2 2p6
d. 1s2 2s2 2p6 3s1
a
Which of the following represenrts an electron configuration that corresponds to the valence electrons of an element for which there is an especially large jump between the second and third ionization energies?
a. 1s2
b. 1s2 2s2
c. 1s2 2s2 2p2
d. 1s2 2s2 2p6
b
Between K2O, Na2O, and Li2O, which would have the highest percentage Oxygen if all the samples were pure?
Li2O
What is the average atomic mass of this isotope?
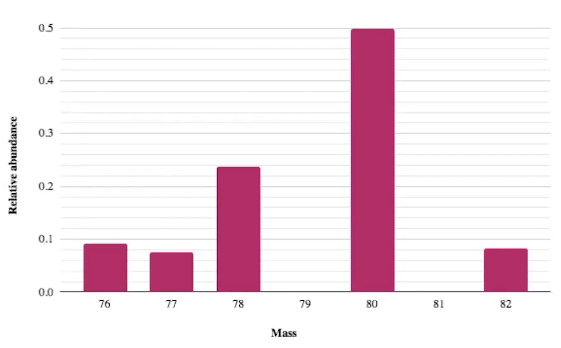
78.57 g
A 5.0 g sample of MgCl2 may contain measureable amounts of other compounds as impurities. Which of the following quantities is (are) needed to determine that the sample is pure MgCl2?
a. The color and density of the sample
b. The mass of Mg in the sample only
c. The number of moles in the Cl in the sample only
d. The mass of Mg and the mass of Cl in the sample
d
Which electron configuration represents the best ground state configuration for an atom of selenium?
a. 1s2 2s2 2p6 3s2 3p4
b. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 5s2 4p4
c. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
d. 1s2 2s2 2p6 3s2 3p6 4s2 3d10
c
If Na reacts with Chlorine to form NaCl which of the following elements reacts with Na to form an ionic compound with the same ratio of elements?
a. N
b. O
c. F
d. Ne
c