What equipment is used to measure the mass of a solid?
Electronic Balance
BONUS: TRIPLE POINTS
Which of these would be best to hold a test tube over a fire?
a)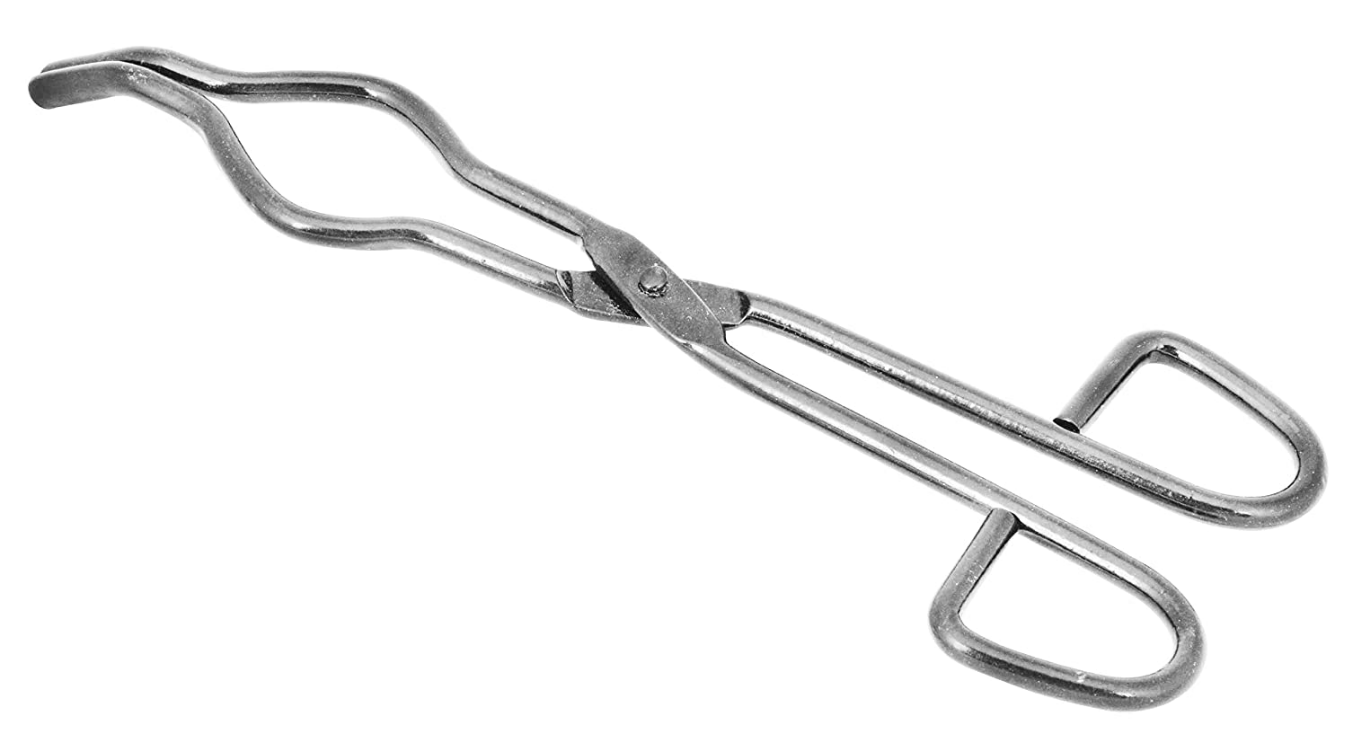
b)  c)
c) 
B) the gold test tube tongs, they are the most secure, clamps more of the test tube
With A, they could slip through, with C that's a wrench and it could break the tube

What subatomic particle is the identity of the element based on?
Protons (atomic number)
Bonus DOUBLE POINTS: Select the two items that would not be appropriate to wear in the lab
a) Jeans
b) Tshirt
c) Scarf
d) Hair tie
e) Slides
C and E
Bonus Equipment: DOUBLE POINTS
What is this equipment used for?
To hold chemicals that release dangerous fumes/gases, vents out the fumes
What is the first thing you must do if you break glassware in lab?
Tell a teacher
Why did the model of the atom change over time?
Where are the three subatomic particles located (All or nothing!)
Protons and neutrons in the nucleus, electrons outside of the nucleus (orbitals, shells, cloud)
Look at the isotope symbol below. How many protons does this atom have?

6
Which of the following are isotopes? (select two)
a) 6 protons 6 neutrons
b) 7 protons 6 neutrons
c) 6 protons 7 neutrons
d) 5 protons 7 neutrons
a and c (same protons different neutrons)
What do you use to measure the volume of liquids?
a)Beaker
b)Test tube
c)Erlenmeyer flask
d)Graduated cylinder
d) Graduated cylinder
Which scientist developed the nuclear model?
a) Dalton
b) Thomson
c) Rutherford
d) Bohr
e) Democritus
C) Rutherford
What are the charges of the three subatomic particles? (All or nothing!)
Protons +1
Neutrons 0
Electrons -1
Name an element that has the same properties (qualities) as sulfur.
Oxygen, selenium, tellurium, polonium, livermorium (any element in the same GROUP)
In terms of subatomic particles, isotopes have the same number of ______________ and different number of ____________, which means they have different _________.
Protons, neutrons, masses
When is it appropriate to leave a lit bunsen burner unattended?
Never
Which scientist is credited with the discovery of the electron and developed the plum pudding model?
a) Dalton
b) Thomson
c) Rutherford
d) Bohr
e) Democritus
JJ Thomson
What are the relative masses of the three subatomic particles?
Protons 1
Neutrons 1
Electrons 0
Which element has 79 protons?
Au (Gold)
What is the most abundant isotope for Krypton?
Krypton - 84
How much water is there in the graduated cylinder below?
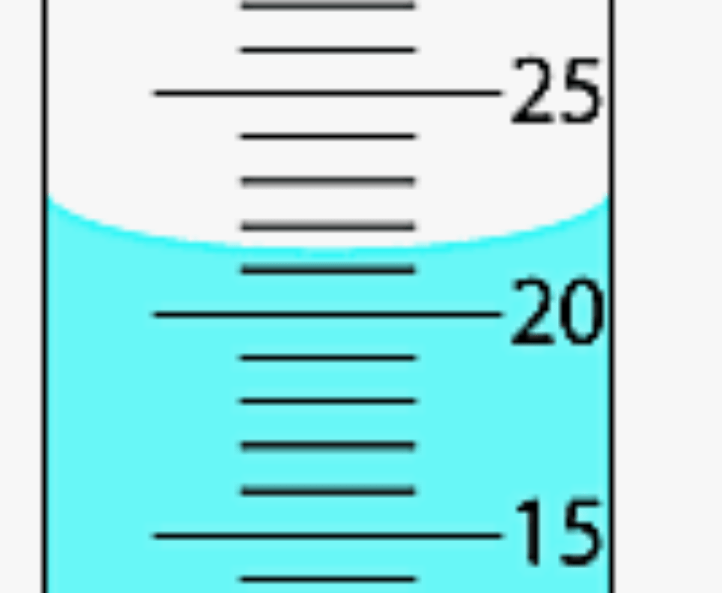
21.5 mL (or 21.6 mL, 21.4 mL)
How did Bohr refine Rutherfrod's model of the atom?
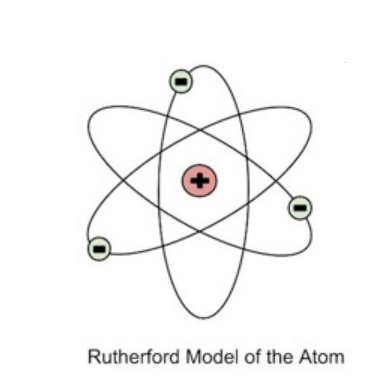
Why is Bohr's model not completely correct/not in line with current evidence?
1) He said that electrons orbited the nucleus like planets in the solar system
2) We can never fully know the exact location of electrons the way Bohr's model shows them
An atom has 3 protons, 3 neutrons, and 2 electrons. What is its charge?
Part 1: What element is in the same period as lithium?
Part 2: Will these elements have the same qualities in common?
Beryllium, Boron, Carbon, Nitrogen, Oxygen, Fluorine, Neon
NO, only elements in the same group have a lot in common
What is the average atomic mass of an element whose percent abundance is 99.1% for the mass of 12 and 0.9% for the mass of 13. Round to the nearest 0.01.
Bonus for everyone: What element is this?
12 (.991) + 13 (0.009) = 12.01 amu
Bonus: Carbon because carbon has an atomic mass of 12.01 (periodic table)
How much liquid is in the graduated cylinder?
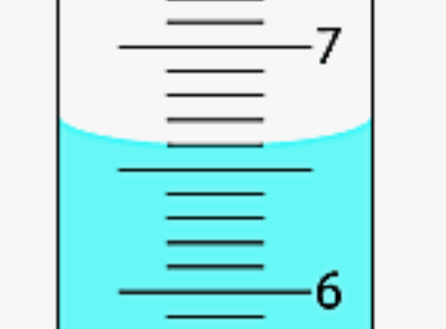
6.60 mL (the 0 is necessary!)
What are the two key discoveries of Rutherford's gold foil experiment?
1)atoms of made of MOSTLY empty space
2)atoms have a dense, positively charged center called the nucleus
An atom has 5 protons, 6 neutrons, and 7 electrons. What is its mass? What is its charge?
11 amu
-2 charge
Look at the isotope symbol below. How many neutrons would an atom of this isotope have?
8 neutrons (subtract the mass - the atomic number)
A scientist discovers an element. Two isotopes of this element is exist. 60% of the isotopes have a mass of 35.112 amu and the rest have a mass of 36.009 amu. What is the average atomic mass of this element? (round to the nearest 0.01 and include unit)
35.112(0.60) + 36.009(0.40) = 35.47 amu