Define "kinetic energy".
The energy of motion.
What equation is used for Boyle's law?
P1V1 = P2V2
What equation is used for Charles's law?
V1/T1 = V2/T2
What equation is used for Avogadro's law?
V1/n1 = V2/n2
Which gas law applies? Eve collects 110 liters of gas at 237 K. Later, the volume of the gas he collected was measured at 178 liters. What is the new temperature of the gas?
Charles's Law
What unit for temperature is required in all gas law equations?
Kelvin (K)
According to Boyle's law, when pressure decreases volume _____________________.
Increases!
Describe Charles's law using this illustration.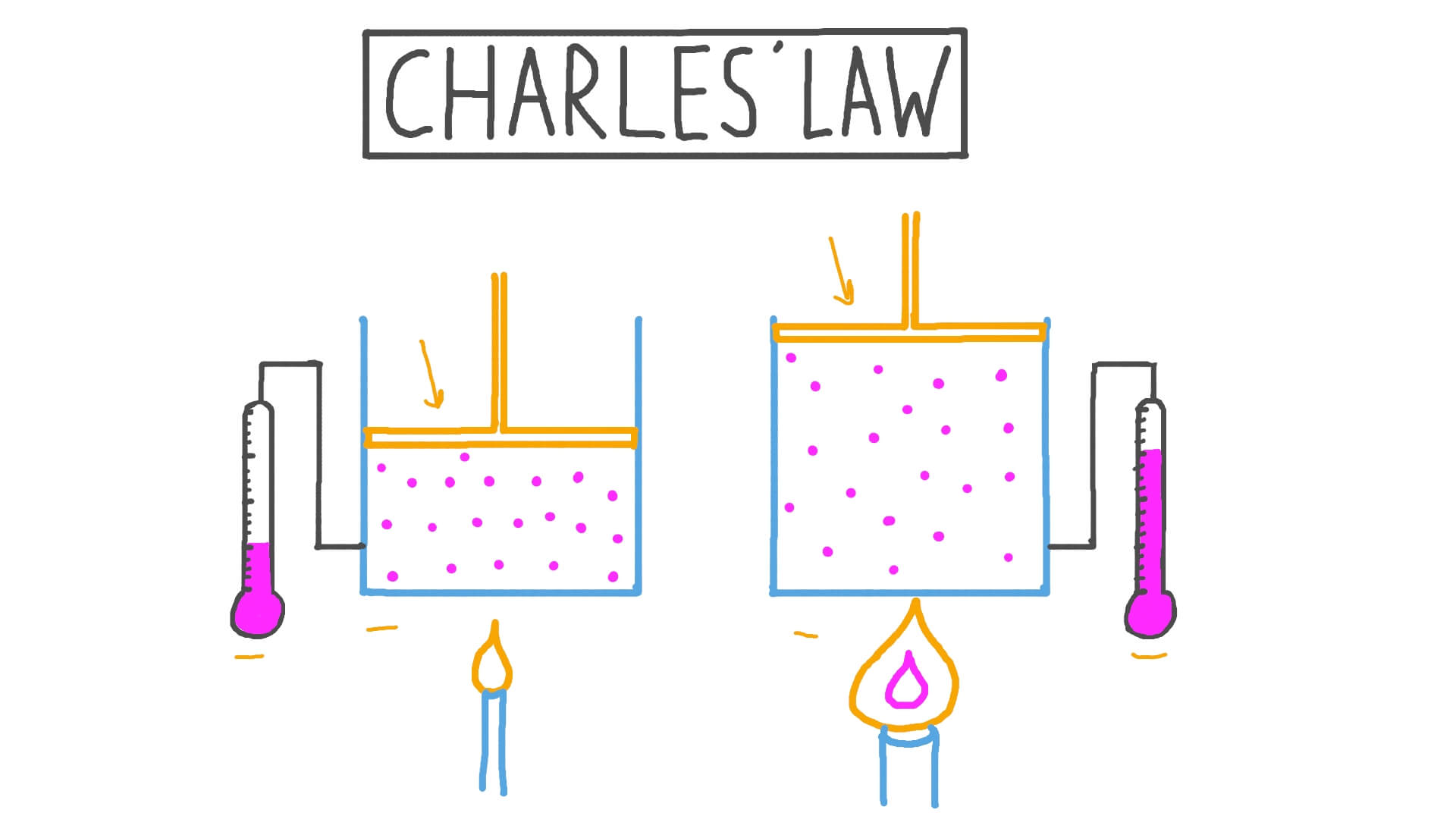
As temperature increases, volume increases.
According to Avogadro's law, when number of particles decreases volume _____________________.
Decreases!
Which gas law applies? Frankie subjects 17.3 liters of gas, collected at 196 kPa of pressure, to an environment that decreases the pressure to 112 kPa. What will her new volume be?
Boyle's Law
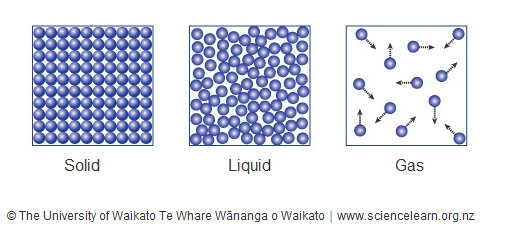
Represent the three states of matter using particle models.
Is the relationship between pressure and volume in Boyle's law direct or inverse?
Pressure and volume have an inverse relationship. As one variable increases, the other decreases.
Draw a graph that represents Charles's law.

Draw a graph that represents Avogadro's law.
Which gas law applies? Eeli heats 4.1 mol of helium gas until his sample of gas reaches a pressure of 178 kPa and a volume of 16 L. What temperature did Eeli heat his sample of gas to?
Ideal Gas Law
What happens to the kinetic energy of the particles in a substance when temperature decreases?
Kinetic energy decreases.
In this problem what is your unknown? "Christina subjects 2.3 mL of gas to an environment that increases the volume to 5.9 mL and a pressure of 1.5 atm. What was the original pressure of the gas?"
P1
In this problem what is your unknown? "Sam subjects 12.1 L of gas at 41 °C to an environment that increases the volume to 18.6 L. What is the new temperature of the gas?"
T2
In this problem what is your unknown? "Claire has a balloon filled with 5.1 mol of gas that occupies 12 L. She fills the balloon until it has 6.3 mol of gas. What is the new volume of Claire's balloon?"
V2
Which gas law applies? A 14 mL sample of gas is heated to 296 K at a pressure of 300 kPA. How many moles of gas are in the sample?
Ideal Gas Law
Convert 3,182 mL to L using the format reviewed in class.
3,182 mL/1 * 1 L/1000mL = 3.182 L
SOLVE! Myron subjects 13.3 liters of gas, collected at 196 kPa of pressure, to an environment that decreases the pressure to 112 kPa. What will his new volume be?
23 L
SOLVE! Alex subjects 200 L of gas to an environment that decreases the volume and temperature to 125 L and 17 °C. What was the original temperature of the gas?
464 K
SOLVE! A sample at 22°C contains 1.5 mole of a gas. If the number of moles of gas is increased to 3.5 moles and volume increases to 11.6 L, what was the initial volume of the gas?
5 L
Which gas law applies? A mixture of oxygen and helium gases exerts a total pressure of 18 atm. If the partial pressure of the oxygen (O2) is 12 atm, what is the partial pressure exerted by the helium (He)?
