Define chemistry
The study of matter and its properties
Graduated Cylinder
Ice Melting
Physical change
How many protons are in Bromine?
35 protons
What did Rutherford discover about the atom?
The nucleus!
How many cm are in 47.9 in?
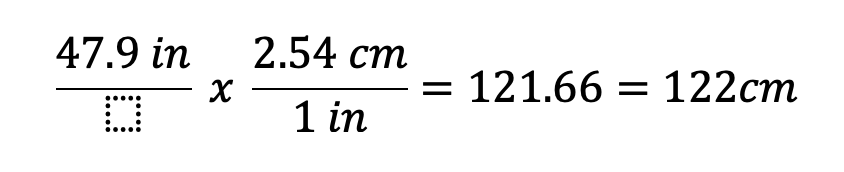
Calculate the molar mass of NaCl.
58.44 g/mol
Classify the following as a substance or mixture. If it's a substance classify it as an element or compound. If it's a mixture classify it as homogeneous or heterogeneous.
IceDefine accuracy
Crucible & lid
Mixing baking soda and vinegar
Chemical change
How many neutrons are in Polonium-210?
126 neutrons
Who came up with the original idea of atoms?
Democritus
How many mm in 17 ft?
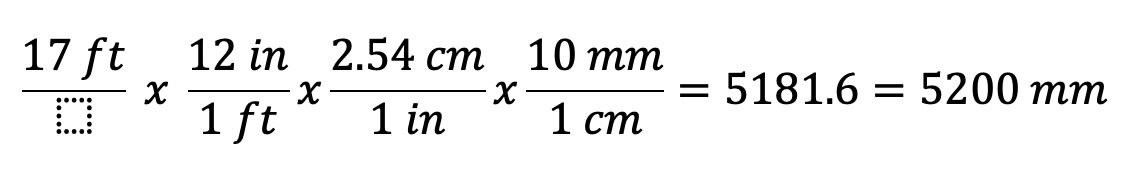
Calculate the molar mass of ethanol (C2H6O).
46.08 g/mol
Classify the following as a substance or mixture. If it's a substance classify it as an element or compound. If it's a mixture classify it as homogeneous or heterogeneous.
Chocolate Chip Cookie
Heterogeneous Mixture
Define proton
A positive subatomic particle located in the nucleus that determines the identity of the atom

Watch glass
Cracking eggs
Physical change
How many electrons are in an atom of Scandium?
21 electrons
What is the currently accepted model of the atom?
The Schrodinger model where electrons are located in particular areas called clouds.
How many meters per second is 23 miles per hour?
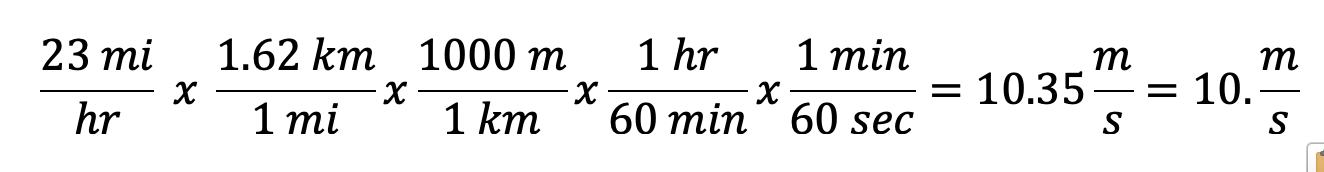
How many grams are in 12.6 moles of H2O?
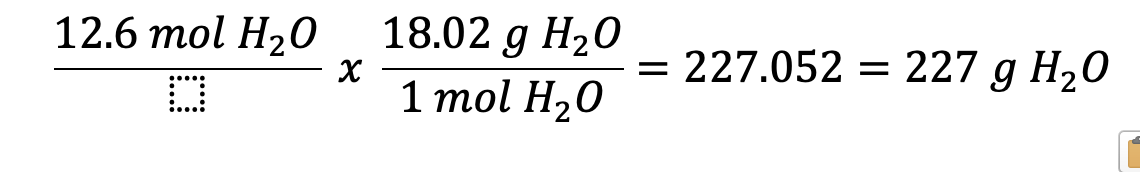
Classify the following as a substance or mixture. If it's a substance classify it as an element or compound. If it's a mixture classify it as homogeneous or heterogeneous.
Brass
Homogeneous mixture
Define intensive property
A property that does not depend on the amount of matter present.

Dropper
Baking a cake
What element has 117 protons?
Tennessine
Draw JJ Thomson's model of the atom.

A car is traveling 28 miles per hour, how many meters will it travel in 45 seconds?
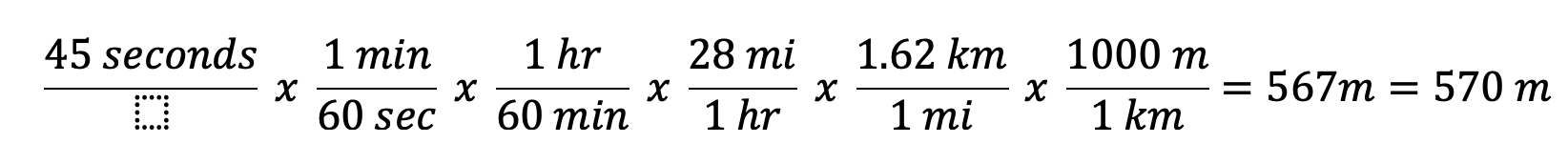
How many atoms are in 6.74 moles of Na?
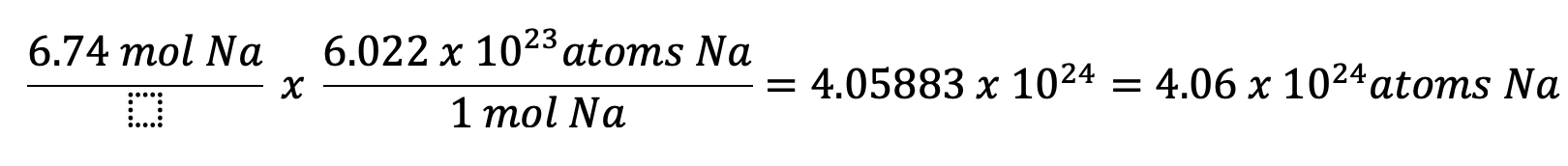
What are the steps of the scientific method?
Question/Observation, Hypothesis, Experiment, Analyze, Draw conclusions
Define law of conservation of matter
Matter cannot be created or destroyed.
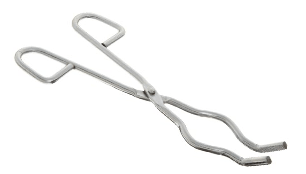
Crucible Tongs
Chemical change
Write in isotope symbol notation the element that has 61 protons and 84 neutrons.
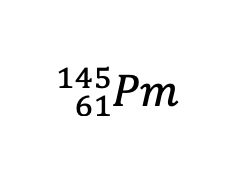
What model of the atom do we most commonly use in a chemistry classroom?
The Bohr model
You discovered a new element called Washtonian. It has 3 isotopes:
Washtonian- 68 (23.98% abundance)
Washtonian-71 (54.13% abundance)
Washtonian-72 (21.89% abundance)
70.50 amu
How many molecules of CO2 are in 7.98 g of CO2?
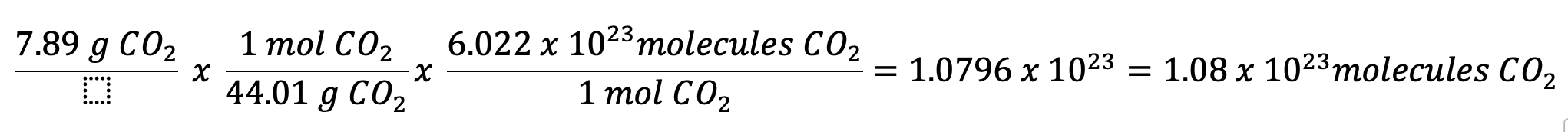
You did a lab and found the density of a substance to be 8.43 g/mL. The actual density is 7.98 g/mL. What is the percent error?
5.6%