True or False. Elements in the same horizontal groups have similar chemical properties.
FALSE
What is the overall charge of a single atom of an element
0
Physical Change or Chemical Change:
Table salt (NaCl) dissolving into water
Physical change
What are the three most common states of Water on earth?
Solid, Liquid, Gas
This type of energy is the energy of movement.
Kinetic Energy
What is the law of the Conservation of Matter?
Heat always flows in which direction on its own?
High temperature to low temperature
What is the atomic number of nitrogen?

7
The three particles of the atom.
Protons, neutrons, and electrons
Physical property or Chemical Property?
Melting point
physical property
What is happening at point "a" ?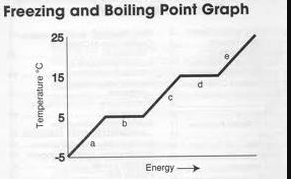
The matter is in the solid state
This type of energy is found in the nucleus of an atom.
Nuclear Energy
DAILY DOUBLE
How are matter and energy related?
matter is just condensed energy proven by e=mc2
The process of heat transferring through direct contact with another solid.
Conduction
What is the grouping of elements, all metals, in the middle of periodic table called?
Transition metals
DAILY DOUBLE
Which of the following is NOT a solution?
- An ingot of brass
- the air you breath
- Chocolate milk
Chocolate milk
Physical Change or Chemical Change:
Pancakes Cook
Chemical Change
Which state of matter has particles vibrating the least?
Solids
What is the difference between Radiant Energy and Electromagnetic Energy?
Radiant is visible light and electromagnetic is not visible light
What energy transformation happens in a windmill?
Kinetic Energy --> Electrical Energy
From which point to which point will heat energy most likely flow to?
A. 10ºC B. 25ºC
C. 30 ºC D. 5ºC
From point C to D.
Elements in the vertical columns on the periodic table have an equal number of these.
Valence Electrons (accept outside electrons)
The Largest area of an atom
The Electron Cloud
Physical property or Chemical Property?
flammability
Chemical
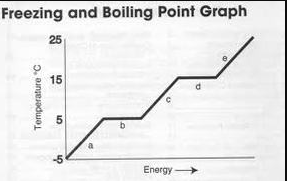
What is happening at point "d" ? Explain your answer in terms of why the matter has NOT yet transitioned to another state.
The matter is vaporizing/boiling/becoming a gas.
This doesn't happen immediately because it takes extra energy to break the bonds between atoms.
Which of the following is NOT a type of energy:
- Electrical
- Electromagnetic
- Radiation
- Sound
Radiation
DAILY DOUBLE
Is this following equation balanced or unbalanced?

unbalanced
How the sun uses light energy to warm the earth
Radiation
If an atom Arsenic has an atomic number of 33 and a mass number of 75, how many protons, neutrons, and electrons should it have?
Protons - 33
Electrons - 33
Neutrons - 42
What is the difference between a solution and a compound?
A compound is matter composed of a single chemical formula.
A solution is two different substance mixed together physically but not chemically
Physical Change or Chemical Change:
Heat changes H2O to steam
Physical
Which do we call the transformation from a solid directly to a gas?
Sublimation
What is the difference between Thermal Energy and Temperature.
Temperature is the kinetic energy of individual particles in an object.
Thermal energy is the average of the kinetic energy of all particles in an object
What coefficient goes in front of H2O?
H3PO4 + 3KOH → K3PO4 + H2O
3
Why does electrical energy, in the form of electrons, flow through a wire to power an object using a battery?
There are a lot of electrons on the negative end of the battery and few on the positive end.
The electrons flow from a high concentration to a low concentration
DAILY DOUBLE
What is the most chemically reactive element?
Francium
What type of energy holds the nucleus of the atom together?
Nuclear
Physical Property, Chemical Property, Physical Change, Chemical Change
Corrosion
Rank the three main states of water on earth in order of increasing thermal energy
Solid --> Liquid --> Gas
How is the Gravitational energy and the kinetic energy of an object related?
When dropped an object loses gravitational energy and gains kinetic energy
In a lab, you combine 120 grams of Al2O3 with 30 grams of H2SO4. After the reaction you have 100 grams of Aluminum sulfate. How much water was produced in this reaction?
50 grams.
What type of heat transfer results in atmospheric circulation?
Convection
Balance the following chemical equation by putting the correct coefficient in from of each compound:
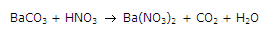
BaCO3 + 2HNO3 --> Ba(NO3)2 + CO2 + H2O