On this picture, which number is the outer core?
EC: What is its composition?
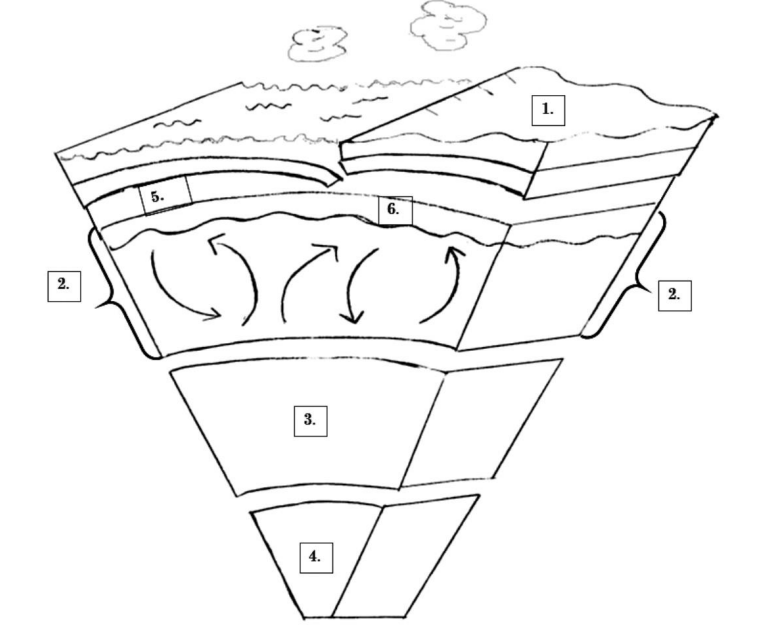
#3 is where the outer core is, and it is composed of liquid metals (Iron and Nickel)
What is the type of thermal energy movement that involves the circulation of fluids?
Convection
What happens to the temperature of a substance as it undergoes a phase change?

The temperature stays constant during a phase change.
If you heat up ice till it melts, what happens to its kinetic energy?
The kinetic energy does NOT increase, because there is no temperature change.
(The PE in the bonds did increase)
How does a lava lamp demonstrate radiation? (There can be more than one answer to this)
Putting your hand around the lava lamp by not touching it and you feel the heat emitting from the lamp.
The lightbulb shining onto the base of the bottle is also transferring energy via radiation.
On this picture, where is magma located?
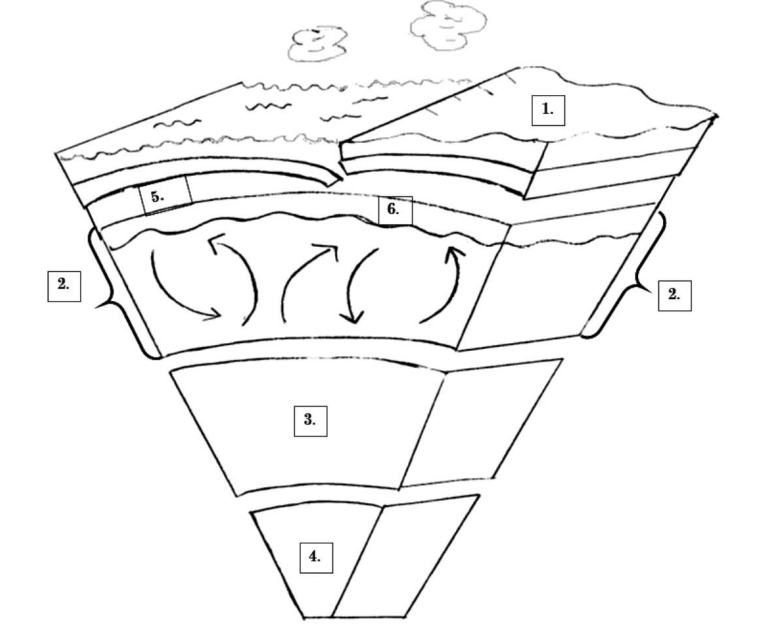
# 2: Mantle
or
# 6: Asthenosphere
What happens during subduction?
This is when two plates collide and one is forced under the other one. For instance, when the oceanic plate collides with the continental plate, the oceanic plate will go under (subduct) the continental plate since it is more dense.
Calculate the heat required to melt 205 g of Ice.
Given that Water's Hvap: 2257 J/g
Water's Hfus: 333.6 J/g
Values can be negative depending on the phase change
Useful Equations
q = mHvap
q = mHfus
Answer is 68,400 J
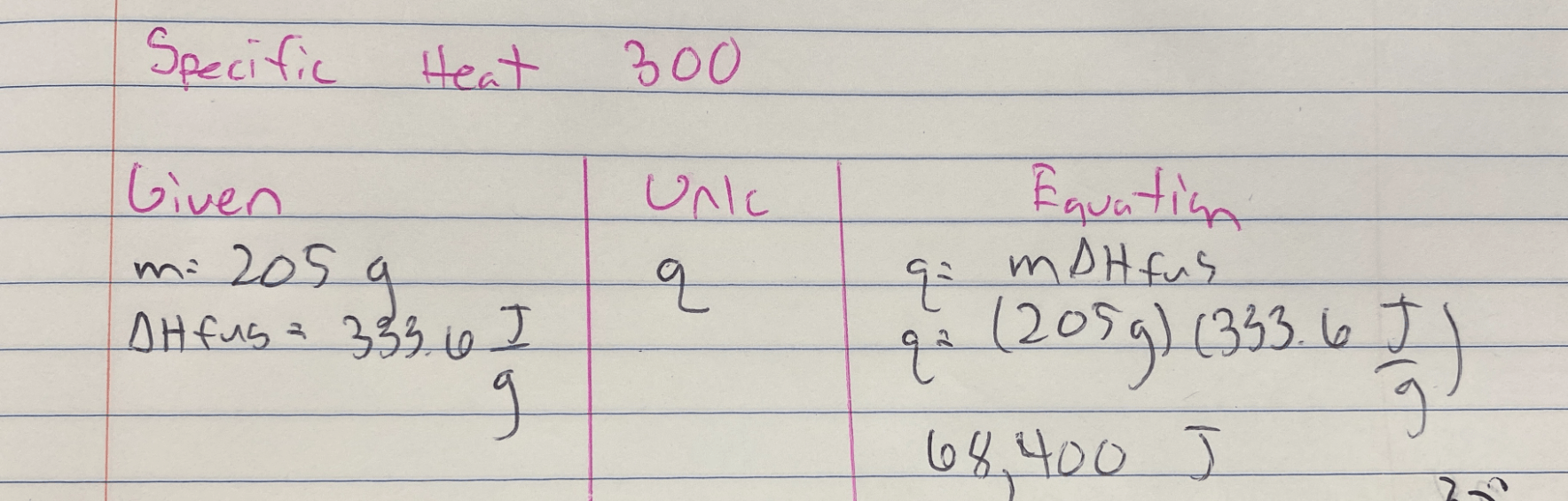
How would you compare the kinetic energy of particles between a gas and a solid?
higher in gas than in solid
quantity of heat needed to change the temperature of 1 g of a substance by 1 degree Celsius
specific heat (c)
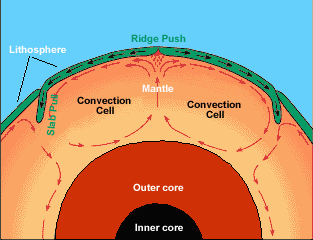
From the picture above, what causes the tectonic plates to move?
This is due to the convection of magma in the mantle/asthenosphere.
This is the Thingvellir fracture zone in Iceland, which is considered a rift. What type of plate boundary do you think this is? How do you know?

Divergent Plate Boundaries, a rift forms, when two plates are moving away from each other.
How much energy being absorbed in Joules is required to heat 50.0 grams of iron with a temperature change of +55°C? The specific heat of iron is 0.444 J/g°C.
Useful Equations
q = mcΔT
Answer is 1220 J
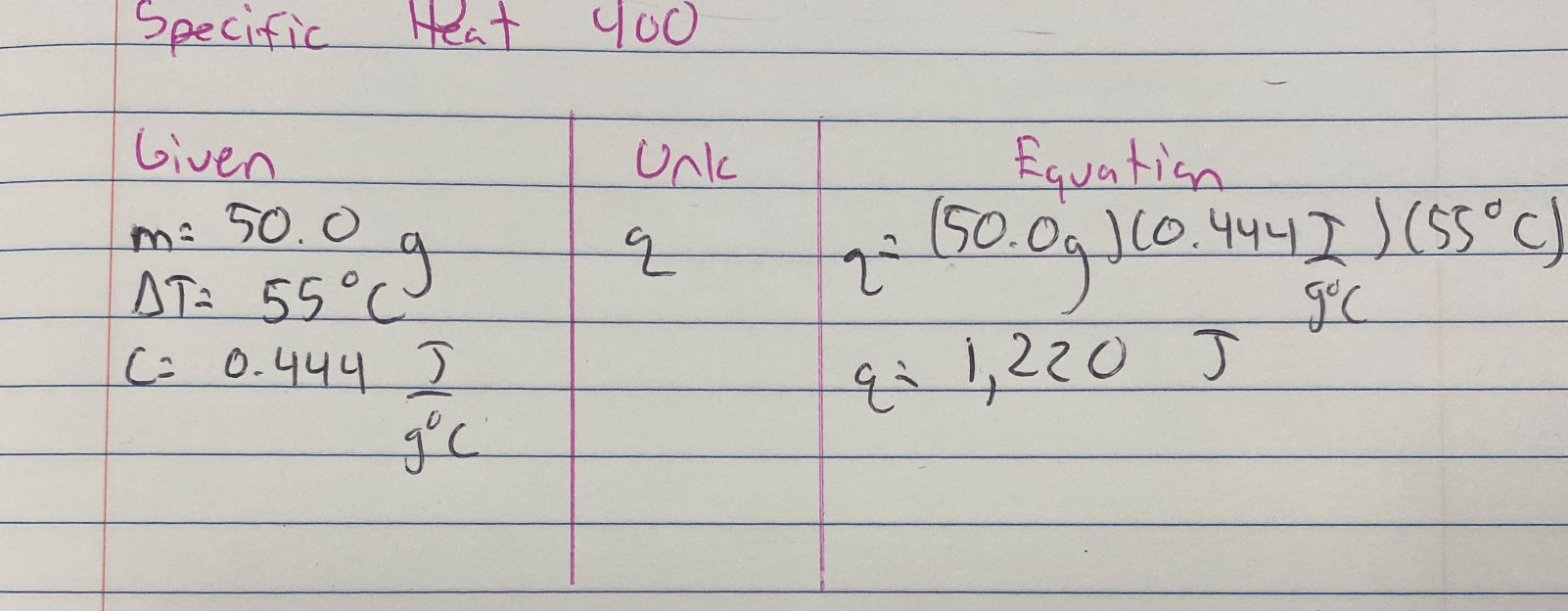
Thermal energy is the measure of the total amount of ____________ energy & ______________ energy within a sample.
kinetic and potential
What are the three types of rocks that we discussed in class and tell me which rocks you can easily find inside a volcano?

These rocks are:
1. Igneous
2. Sedimentary
3. Metamorphic
Igneous rocks are common inside a volcano.
Looking at the plate map above, how is the Pacific Plate moving relative to the Nazca Plate? What is this type of boundary called?
The Pacific Plate is moving away from the Nazca Plate. This is a type of divergent boundary.
A volcano was discovered in an uncharted island. Based on the analysis, it was characterized as a composite volcano, with a high Silica and Felsic Composition, like Granite has. How will this volcano erupt?
explody
A liquid absorbs 65660J of energy when 391g of the liquid changes from 11.5oC to 68.7oC.
What is the liquid's specific heat?
2.94 J/goC
you need to rearrange the mcat formula to be
c=q/(m delta T)
sorry, I can't type a triangle, so that is what the delta means
What is the rule for the direction of any spontaneously movement of thermal energy when objects are brought into thermal contact?
Heat moves from a hotter object to a cooler object.
This is the 2nd Law of Thermodynamics
When you place a piece of ice on a piece of plastic and a piece of aluminum that are both the same temperature, you notice that the ice melts faster on the aluminum. What property of the aluminum is responsible for this phenomenon?
Aluminum's thermal conductivity is higher than plastic's!
Within the Atlantic Ocean, the Eurasian Plate and the North American Plate are touching. What kind of landform will you see at this border?
It will be a mid ocean ridge system due to a divergent boundary plate interaction.
Provide an example of how the conduction of heat occurs in the Earth's layers.
Since the earth's layers are touching each other, the heat from one layer can be transferred to another layer. Also, heat works its way through the solid layers via conduction (crust/lithosphere, mesosphere, inner core)

80.0g of water at 21oC is heated to boiling, then completely boiled off. How much energy was absorbed by the water molecules?
cwater = 4.18 J/goC, Hfus of water is 334 J/g, Hvap of water is 2260 J/g.
21oC --> 100oC: we use mcat to find
q = 26418J
boiling: we use q=mHvap to find
q = 180800J
then we add them:
total q = 207200J
Give examples of two objects and let me know which of these have a greater thermal energy (There can be many possible examples for this)
2 cubes of ice vs Glacier
Glacier has a larger thermal energy as there are more ice particles on this than the two cubes of ice.
PS- I don't like this question and one like it will not be on the test. -carter
What kind of curve is the illustration below?
This is a cooling curve since heat is leaving the system.