What are the physical states of matter?
The physical states of matter are solid, liquid, and gas.
What is the process called when a liquid turns into a solid?
This process is called freezing.
What are physical properties?
Physical properties are how observe or measure matter.
*double points*
What is a mixture?
A mixture is a combination of two or more ingredients.
F.I.B
Most conductors are what ________________
Most conductors are metal.
Which state of matter has a defiant shape and a defiant volume?
A solid
What process called when a gas turns into a liquid?
It is called condensation.
Which physical property allow energy to flow through matter?
This is conductivity.
Name the two characteristics of a mixture.
A mixture keeps it's physical properties and can be easily separated.
Name 2 non-metal conductors.
Water and Wax
The particles in this picture represents a ...
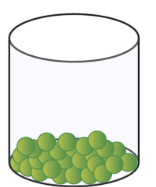
A liquid
double points
What is the process called when heat is added to liquid?
This process is called evaporation.
Why is rubber used to protect the workers feet from the electricity?
Rubber is a good insulator.
What is a solution?
A special kind of mixture of two or more ingredients.
Is glass an electrical conductor or a thermal conductor?
Glass is a thermal conductor.
*add half points of leading team*
Which two physical states takes the shape of the containers they are put in?
A liquid and a gas.
F.I.B
When water is heated to 100 degrees C this called the ___________________
Boiling point
double points
Name all the physical properties you focused on this year.
5 senses, conductivity, states of matter, volume, magnetism, mass, density, and solubility.
double points
Name the 3 characteristics of a solution.
A solution loses it's physical properties, not easily separated, and dissolves evenly.
*double points*
Which of these items have particles too small to see?
a. water/stones
b. fruit salad
c. salt and water
Which state of matter has the particles moving around quickly at a fast pace all over the place.
A gas
A wax candle changes states how many times after being lit and burned out?
It changes two times.
Solid to Liquid to Solid.
What is the difference between the solute and the solvent?
The solute is the being dissolve and the solvent is doing the dissovling.
Name 3 ways to separate a mixture.
Name the only way to separate a solution.
Hands, magnet, funnel, strainer, tweezers.
Evaporation
What is the formula for water displacement?
Final volume - Original volume = Water Displacement