4.078 + 67.3999
What is 71.478?
The metric unit for volume.
What is a liter?
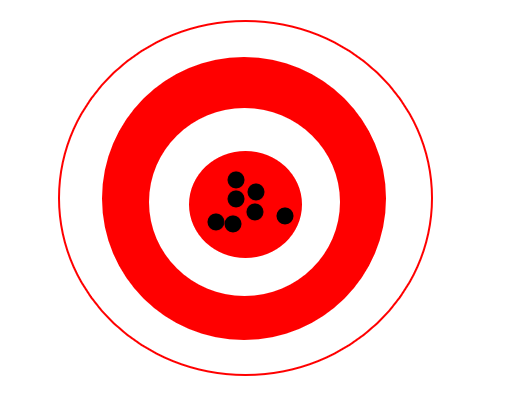
Draw a target with data points that are precise and accurate

1.93 x 8.721
What is 16.8?
A cube of aluminum measures 2.5 cm x 3.7 cm x 9.1 cm and has a mass of 227 grams. Find density.
What is 2.7 g/cm3?
Convert 10 milliliters to liters.
What is 0.01 mL?
Draw a target with data points that are precise, but not accurate


The number of Sig Figs in 1.4007 x 10^3
What is 5
(0.0104 x 10030) + 3.9
What is 108?
Find the mass of 25.0 mL of copper (d=8.96 g/mL)
What is 224 g?
Convert 62 seconds to milliseconds.
What is 62000 ms?
Convert 1.02 x 10^4 into standard notation.
What is 10200?
Round 456 to 1 significant figure
What is 500?
(2.0000 - 1.00) / 2.0
What is 0.50?
What is the density of the dino if it has a mass of of 0.500 g?
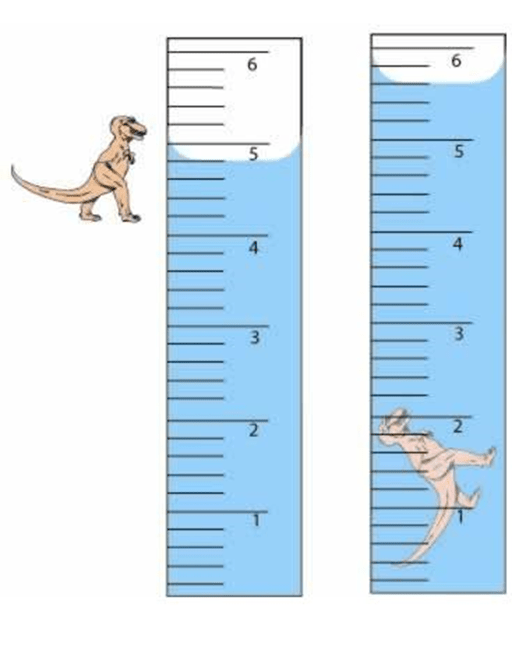
What is 0.63 g/mL?
Convert 53 centigrams to decigrams.
What is 5.3 dg?
Convert 0.000345 to scientific notation.
What is 3.45 x 10^-4?
Round 1400.0030 to 4 significant figures
What is 1400. or 1.400 x 10^3?
(5.0469 + 3.04) / 9
What is 0.9?
The mass of an object has a density of 2.7 g/mL. Find volume if the mass of the object is 12.8 g
What is 4.7 mL?
Convert 400 milliliters to kiloliters.
What is 4 x 10^-4 kL?
A student measures the mass as 3.5 grams but the true value is 4.2 grams. This is the percent error.
What is 20%?