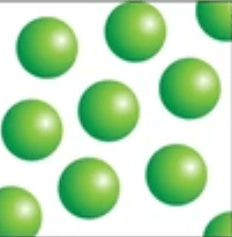
Element
Cracking an egg
Physical Change
A hot body releases thermal energy into the surroundings through this.
Radiation
The smaller the crystals, the more ________ ______ exposed.
surface area
A teaspoon of dry coffee crystals are an example of
Solute
As the temperature decreases, the particles move __________.
slower
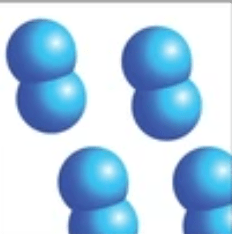
Element
Cutting hair
Physical Change
Heat transfer between two surfaces (particles) that touch each other.
Conduction
The smaller the crystals, the __________ they dissolve.
Water is an example of a
Solvent
When thermal energy is ____________, temperature decreases.
removed
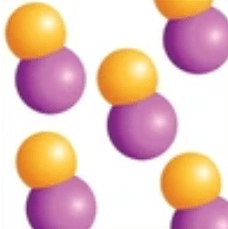
Compound
Burning sugar
The particles or objects exchanging heat do not change their positions.
Conduction
3 affects the rate of dissolution of a solute are
-Temperature
-?
-Agitation
Surface Area

Solute
increases
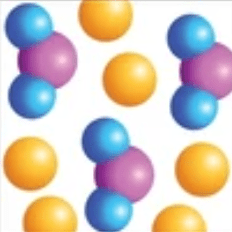
Mixture of elements and compounds
Breaking a glass
Physical Change
Feeling warm while standing by a fire.
Radiation
The larger the crystals, the ___________ it takes to dissolve.
longer
Sugar is an example of a
Solute
As the temperature ______________, the particles move slower.
decreases
a combination of two or more different elements chemically combined.
Compound
Rotting food
Chemical Change
Heat gets distributed in gases and liquids by this process.
Convection
3 affects the rate of dissolution of a solute are
-?
-Surface area
-Agitation
Temperature
A mixture of a solvent and a solute
Solution
As the temperature ______________, the particles move faster.
increases
Chemicals that are pure substances represented by symbols that have at least one capital letter.
Element
Change in color, new substance formed, not easily reversible are properties of what?
Chemical Change
Heat gets transferred from one end of a solid to another through this.
Conduction
How do using smaller salt crystals affect the rate of making a salt solution in water?
Smaller crystals increase the surface area exposed to the solvent and speed up dissolving.
Salt is an example of a
Solute
When thermal energy is removed, temperature ______________.
Decreases
made up of one type of atom
Change in shape, no new substance formed, can be easily reversed are properties of what?
Physical Change
Touching a hot bowl of soup
Conduction
3 affects the rate of dissolution of a solute are
-Temperature
-Surface area
-?
Agitation
What dissolves the particles (powder)?
Solvent
What most likely occurs when thermal energy is added from a liquid?
The temperature of the liquid increases.
Compounds
Two colorless liquids were mixed, and a color change occurred when a new substance was formed. This is an example of a what?
Chemical Change
Can take place without any medium between the source and the destination
Radiation
What is the concentration in percent of 150 mL total of solution that contains 30 g of solute?
20%
Solvents dissolve what
Solutes
When thermal energy is __________, temperature increases.
Elements
What kind of change is this, and why?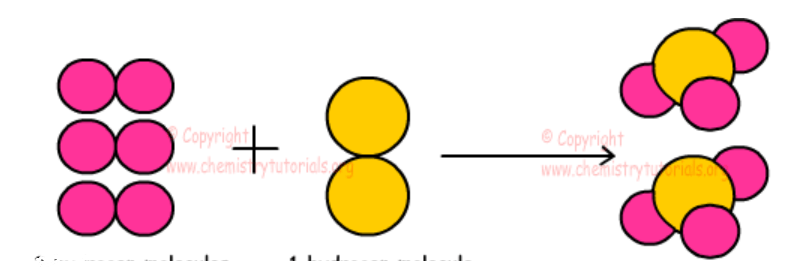
Chemical Change, because a new substance was created.
Warmer portions of the substance rise, allowing cooler portions to come down and take their place.
Convection
A saline solution is made by mixing 25 g of salt (NaCl) dissolved in 250 g of water. What is the percent concentration of salt in the solution?
9%
Chocolate Milk
Solution of chocolate powder and milk
As the temperature increases, the particles move __________.
Faster
Is O2 an element or compound? EXPLAIN why.
A new substance created with different properties was produced.
Chemical Change
After playing outside on a cold day, you decided to go inside. When you go inside, you begin to warm up. How does heat transfer apply?
Heat flows from the object with the higher temperature to the object with the lower temperature
A solution contains 50mL of ethyl alcohol mixed with 150 mL of water for a total of 200 mL of solution. Calculate the concentration of the solution.
25%
Particles (powder) that get dissolved
Solute
What most likely occurs when thermal energy is removed from a liquid?
The temperature of the liquid decreases.