True or False: When you read measurements you need to go to one place beyond what the device measures.
True
Accuracy
What is the equation for density?
Density= Mass/ Volume
True or False:
Positive exponent= larger
Negative exponent = smaller
True
A ratio of equivalent measurements used to convert a quantity from on unit to another is know as...?
a. conversion factor
b. accuracy
c. dimensional analysis
a. conversion factor
What is always constant and stays the same in the experiment is known as the:
a. Independent Variable
b. Dependent Variable
c. Control
c. Control
Describe the accuracy and precision.

What is the correct way to solve for volume is I have a mass of 4.5 grams and a density of 7.85 g/mL.
a. 7.85 g/mL / 4.5 grams
b. 4.5 grams / 7.85 g/mL
c. 4.5 grams x 7.85 g/mL
b. 4.5 grams / 7.85 g/mL
Which of the following is NOT in scientific notation?
a. 8.56 x 103
b. 8.56 x 10-3
c. 85.6 x 103
d. 8.56 x 101
C. 85.6 x 103
How many miles is equivalent to 68,640 feet?
(1 mile = 5280 feet)
13 miles
If a box weighs 45 kilograms, what is the mass of the box in grams. (K H D u d c m)
45,000
This picture represents:
High Accuracy, Low Precision
What is volume displacement?
To find the volume of a irregularly shaped object. Volume after- volume before.
The coefficient should be a number greater than ______ and less than ______?
One; Ten
There is a string that is 28 ft long. This is how many millimeters long the string is.
(1ft=12 in; 2.54cm=1in; 10mm=1cm)
8534.4mm
A study was done to find if different tire treads affect the braking distance of a car. What is the Independent variable?
Tire treads
What is the percent error of an experimental value of .156 cal/g and accepted value of .185 cal/g.
15.68%
Diamonds have a density of 3.5 grams/cm3. How big is a diamond that has a mass of 0.2 grams?
0.06 cubic centimeters
Convert 5.245 x 106 to standard form.
5,245,000
6240 hours is how many days?
260 days
Record the measurement in millimeters.
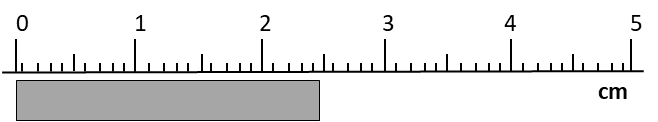
24.5 mm
Data: 1.11, 1.12, 1.14, 1.15, 1.24, 1.27, 1.33, 1.88, 1.67, 1.45, 1.22
Actual - 1.34
Percent Error? Accuracy?
Percent Error: 0.75% or 1.08 %
Accurate: Yes
An irregularly shaped stone was dropped in a graduated cylinder containing a volume of water equal to 7 mL. After the stone was dropped, the water rose to 18 mL. What is the volume of the stone?
11 mL
Write the number 0.0000452 in scientific notation.
4.52 x 10-5
How many millimeters are in 1 kilometer? Record your answer in scientific notation.
1.0 x 106