Plants rely on this property of water to pull water upward from roots to leaves.
What is capillary action (cohesion + adhesion)?
A student claims fertilizer improves plant growth is an example of this.
What is hypothesis?
All living things are made of these.
What is cells?
Smallest unit of matter of all elements.
What is an atom?
A substance with a pH of 3 is considered this.
What is an acid?
This property of water is why sweating cools the body (hint: This is NOT high heat capacity).
What is high heat of vaporization?
The part of an experiment that stays the same for all groups.
What is the control?
All living things must maintain stable internal conditions, a process called this.
What is homeostasis?
This type of bond forms within the water molecule.
What is a covalent bond?
A substance with a pH of 10 is considered this.
What is a base?
Insects like water striders can “walk” on water because of this water property.
What is surface tension?
In CER, this part is a statement of what you believe or what you're trying to explain.
What is the claim?
This characteristic explains how species change over generation.
What is evolution?
The particle with a positive charge, found in the nucleus.
What is a proton?
A substance with a pH of 10 is considered this.
What is a base?
Predict what would happen to aquatic ecosystems if ice were denser than liquid water.
What is bodies of water would freeze solid, killing aquatic life?
This combines observations and experiments into a general explanation for a scientific phenomenon.
What is a theory?
Explain why DNA is considered the universal code of life.
What is all organisms use DNA to store and transmit genetic information?
Atoms of the same element with different numbers of neutrons are called this.
What are isotopes?
Write down the formula for a hydrogen ion or proton.
What is H+?
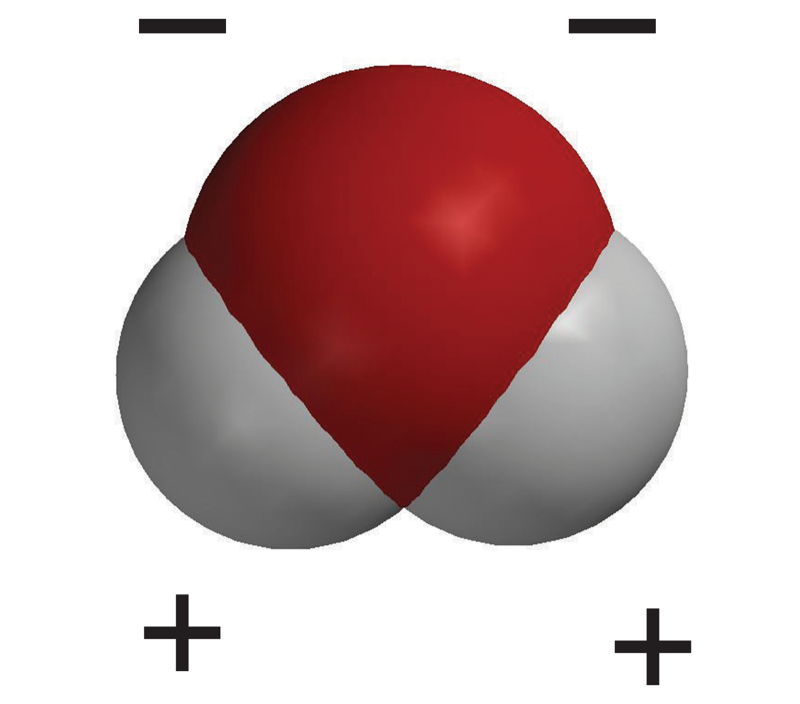
This is why it is important to test only one independent variable in a controlled experiment.
What is to ensure the experimental outcome is clearly due to one identifiable factor (cause-effect relationship)?
This is why viruses are not considered living.
What is they lack cells/metabolism/reproduction on their own?
The number of protons in an atom is called this.
What is the atomic number?
Write down the formula for a hydroxide ion.
What is OH-?