![]()
How many neutrons are in an atom of lithium (as shown in image)
= 7-3 = 4
Ionic bonds are formed because of the electrostatic attraction between positive and negatively charged ions.
Covalent bonds, however are formed between ____________ and involve the ____________ of electrons.
nonmetals
sharing
Identify the only ion that is NOT polyatomic:
Hydronium
Sulfide
Sulfate
Sulfite
Sulfide
S2-

What happens to liquid water when it vaporizes?
a) It transforms into hydrogen gas and oxygen gas.
b) The intermolecular bonds break and molecules separate from each other.
c) The intramolecular bonds break and molecules separate from each other.
d) The attractive forces between molecules get smaller.
b) intermolecular bonds break
Which of the following are examples of systematic errors?
I Weighing a precipitate on an electronic balance.
II Consistently reading burette volumes from the top of the meniscus.
III Using an incorrectly labelled standard solution in a titration.
a) I and III only
b) I, II and III
c) I and II only
d) II and III only
d) II and III only
The ______________ is the energy required to remove one mole of the most loosely held electrons from one mole of gaseous atoms to produce 1 mole of gaseous ions each with a charge of 1+.
This is more easily seen in symbol terms.
First ionisation energy
(will accept just Ionisation Energy)
The formula for aluminium carbonate
Al2(CO3)3
** note that subscripts and brackets must be used
The shape of water (shown above) is bent, whereas carbon dioxide is linear. How do you account for the large difference in bond angle?
a) Oxygen has two lone pairs; these electron-filled orbitals take up space.
b) The hydrogen atoms attract each other, reducing the angle.
c) The double bonds are responsible for the linear structure; without the double bonds, there is always an angle.
d) Oxygen is bigger and takes up more space than hydrogen, stretching the angle to 180°.
a) Oxygen has two lone pairs; these electron-filled orbitals take up space.
(lone pair repulsion)
Urea is dissolved in water and the temperature decreases. Identify the correct statement.
a) the heat capacity of water has changed
b) this process is exothermic
c) this process is endothermic
d) the enthalpy change was negative
c) endothermic
Which of the following is the best definition of precision?
a) A measure of the reproducibility of a result.
b) A measure of how good an experimenter's technique is.
c) How close a value is to the true value.
d) The accuracy of a measurement.
a) reproducibility
This principle states that no two electrons in the same atom can have identical spin when filling electron orbital diagrams and no more than 2 electrons can occupy the same orbital
The Pauli Exclusion Principle
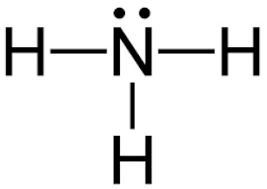
Draw the Lewis dot diagram for ammonia
Draw a balanced equation for the combustion of propane (C3H8)
C3H8+5O2→3CO2+4H2O
************* DAILY DOUBLE ***************
You may bet any of your points (in increments of 300)
i.e. 0, 300, 600, 900, etc. If you do not get the answer right, you will lose that number of points. If you answer correctly you will win those points. (teacher: write the bets on the board)
Here is the question:
In an experiment, 1.2 g of sodium hydroxide pellets, NaOH(s), were dissolved in 100 mL of water at 25°C.
The temperature of the water rose to 27.5°C.
Calculate the heat released, q, in joules (J), by the reaction
q = mass(water) × specific heat capacity(water) × change in temperature(solution)
q = m(H2O(l)) × cg(H2O(l)) × (Tf - Ti)
q = 100 × 4.184 × (27.5 - 25) = 1046 J
What is the correct measurement for this burette reading??
a) 21.4 ± 0.05 cm3
b) 21.61 ± 0.05 cm3
c) 21.6 ± 0.01 cm3
d) 21.6 ± 0.1 cm3
d) 21.6 +/- 0.1 cm3
The scale goes up by 0.2cm3 - the uncertainty will be half of that (0.1cm3) - we can only measure to the nearest 0.1cm3

The element for which this orbital diagram is drawn
Magnesium
Calculate the mass of oxygen in 7.35g CO2
% O by mass: (2 x 16)/44 x 100% = 72.7%
7.35 x 72.7 = 5.35g
How many moles of silicon dioxide are in a 3.5g sample?
0.058 mol
Is the following reaction endothermic or endothermic?
enthalpy reactants = 2083 kJ/mol
enthalpy products = 2003 kJ/mol
delta H = Hproducts - Hreactants
= 2003 - 2083
= -80kJ/mol
This is exothermic.
Using the picture provided below, calculate the retardation factor (Rf) for Protein #2. Give your answer to 2 decimal places
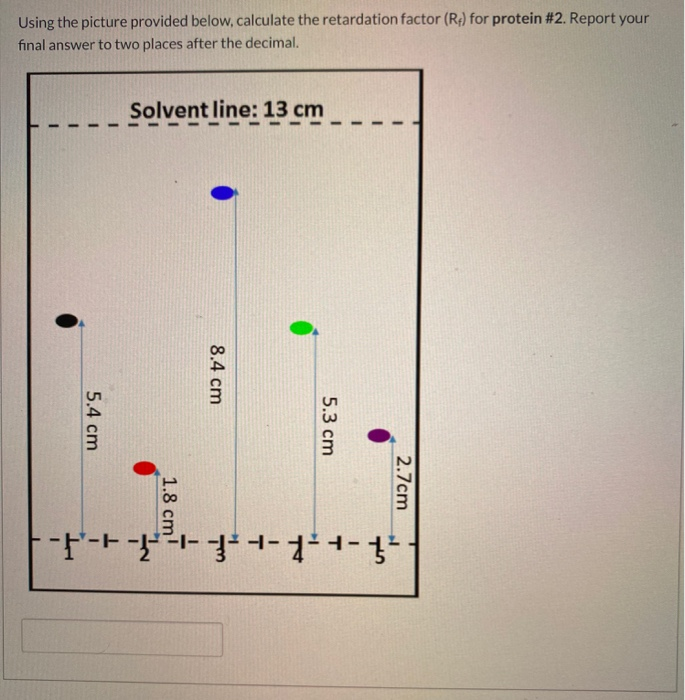
Rf = distance traveled by solute/ distance traveled by solvent
= 1.8/13 = 0.14
In terms of reactivity, how are F- and Ne similar?
Explain.
(must show both for the points)
F- and Ne are both fairly unreactive.
Explanation: They both have the same electron configuration (10 electrons) and a full valence shell.
Copper has 2 naturally occurring isotopes.
Cu-63 has an abundance of 69.17% and a mass of 62.930
Cu-65 has an abundance of 30.83% and a mass of 69.928.
Calculate the average atomic mass of copper
(0.6917 x 62.930) + (0.3083 x 69.928)
= 63.546
For the equation:
2 H2O2 --> 2H2O + O2
If 0.3 moles of hydrogen peroxide react completely, how many grams of oxygen will be produced?
n = 0.3 mol x 1/2 = 0.15
M = 32 g/mol
m = nM = 0.15 x 32 = 4.8g
4.8g of oxygen will be produced
The lead level in a sample of contaminated soil was determined using atomic absorption spectrometry. A 2.0 g sample was dissolved in acid and then diluted to a total volume of 50.0 mL. The absorbance of this solution, determined in a spectrometer set at a wavelength of 218 nm, was found to be 0.20. Several standard solutions of lead were tested under the same conditions and the calibration curve shown below was generated. What is the mass of lead in the original sample?
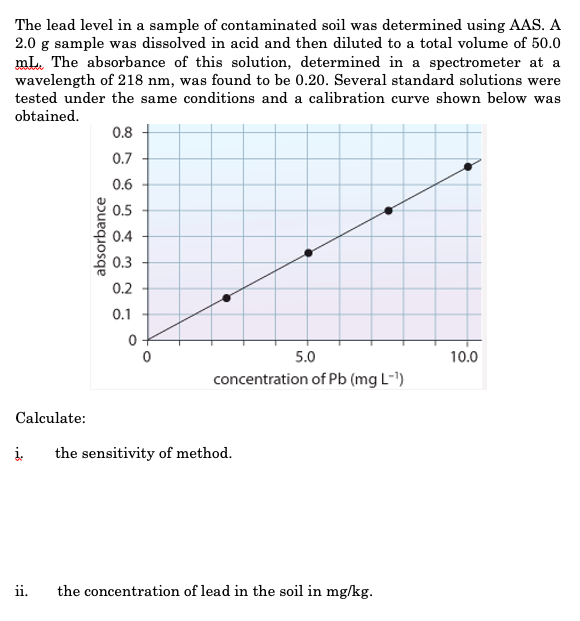
absorbance of 0.2% corresponds to 3mg/L
3mg/L x 0.050 L sample = 0.15mg