Density = Mass / Volume
Which change results in atoms in the reactants rearrange themselves and bond together differently?
Chemical Change
What is the mixture that can be easily separated?
(correct spelling without cheating gives you an extra 100 points!)
Heterogenous Mixture
The law of conservation of mass states:
Mass is neither created or destroyed
What material doesn't have luster and can sometimes conduct electricity?
Metalloid
If two objects have the same volume, but one has more mass than the other, are the densities of the two objects the same or different?
The densities are different because density relies on both mass and volume.
Which piece of evidence best supports that milk spoiling is a chemical change?
A. The milk remains white
B. Solids form in the milk
C. A gas forms which gives the milk a bad odor
D. The milk warms up when left out of the refrigerator
C. A gas forms which gives the milk a bad odor
Is it an element or compound?
A.
B.
A. Compound
B. Element
A student creates a mixture of water and washing soda weighing 10 g and a mixture of water and epsom salts weighing 15 grams. He knows that when he mixes the two substances a chemical change will occur. According to the law of conservation of mass, what should the weight of the products be after he mixes them?
(USE THE CORRECT UNIT OF MEASUREMENT!)
25 g
What type of element is this object?

Metal
Glycerine has a density of 1.26 g/cm3. Saltwater has a density of 0.87 g/cm3.
Which of the following objects will float above glycerine and below saltwater? Select three correct responses.
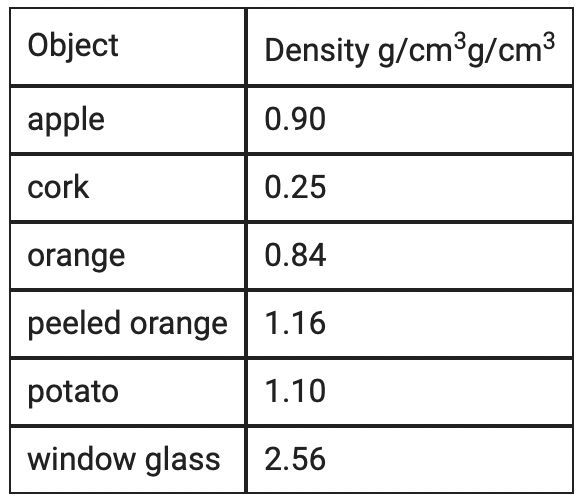
Apple
Orange
Potato
Are the following chemical or physical changes?
Put C or P for each in order.
-Digesting a chocolate bar
-Breaking apart a bar of chocolate
-Melting Butter
- Burning fuel in a car
- Rusting Nail
- Boiling water
C
P
P
C
C
P
Nitrate, NO3, is an important component of many fertilizers.

Use the periodic table to identify the types of atoms that make up NO3.
1 Nitrogen and 3 Oxygens
Drew added yeast and sugar to lukewarm water and placed it in a flask. He stretched a balloon over the opening of the flask. After several minutes, he noticed small bubbles rising along the side of the flask as the balloon slowly began to inflate.
How should the mass of the balloon/flask complex after the reaction relate to the mass of the balloon/flask complex before the reaction?
The mass should be the same since matter is neither created nor destroyed.
What elements and how many atoms are present in this chemical formula?
H3PO4
Hydrogen: 3
Phosphorus: 1
Oxygen: 4
A cube of metal measures 3 cm on each side. It has a mass of 216g What is the density of the metal?
72 g/cm
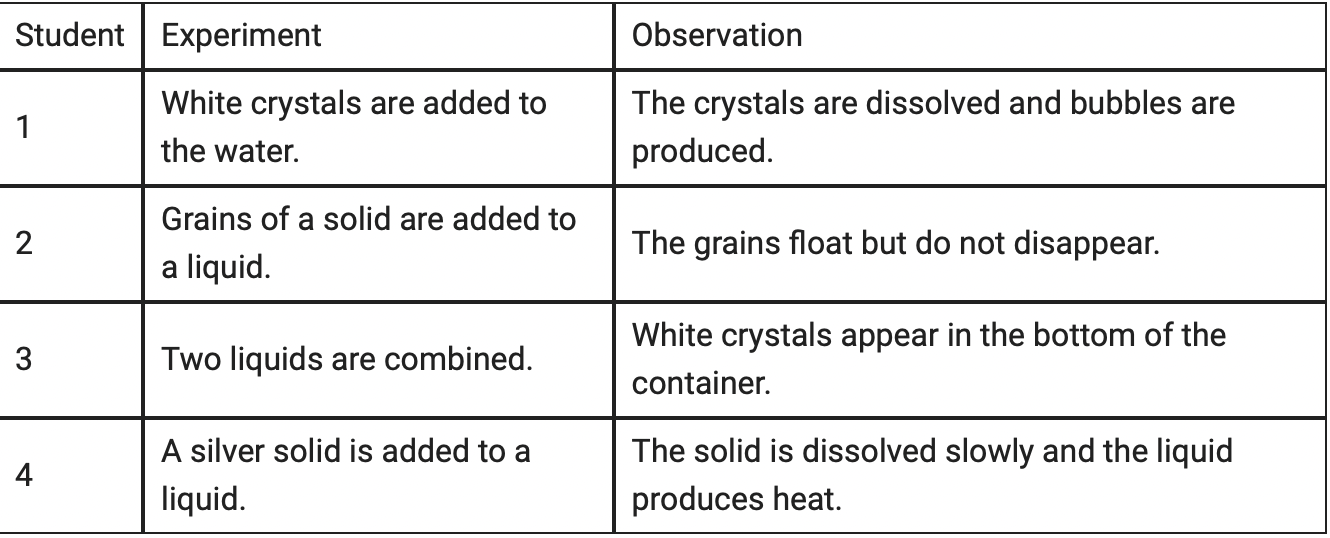
Which of the students' experiments resulted in a physical change?
Student 2
Is it heterogenous or homogenous?
A.
B.
A. Homogenous
B. Heterogenous
Which of these models represent the law of conservation of mass?
Select TWO correct answers.
A.
B.
C.
D.
A and D
This photo shows an element that is often used in electrical wiring.

What are THREE characteristics that this element identified in Part A have that will be useful in electrical wiring?
- Ductility
- Malleability
- Conductivity
A candle will sink in isopropyl alcohol but will float in water.

Why does this happen?
The density of the alcohol is less than the density of water. And the candle's density is in the middle of the water and alcohol.
A student carried out four experiments combining two liquids. The table summarizes the liquids and solids that were combined and the student’s observations.
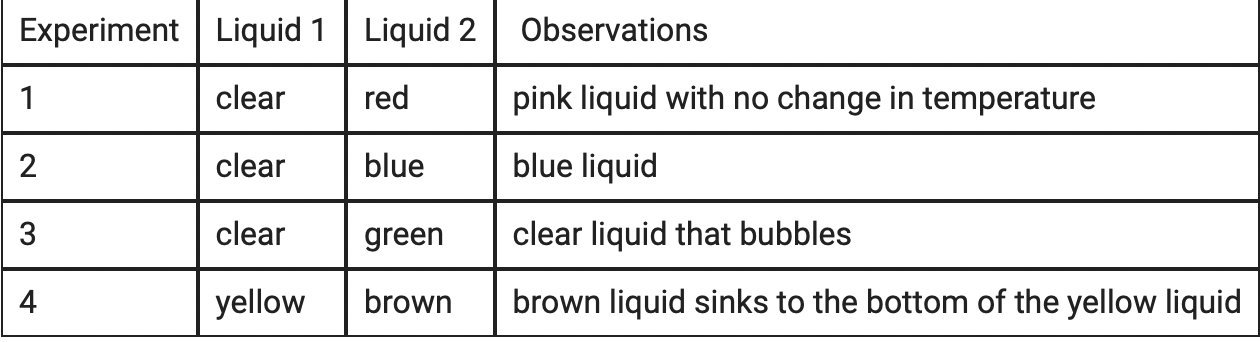
Which experiment is most likely caused by a chemical change?
Experiment 3
The chemical formula for chocolate is C7H8N4O2.
Use a periodic table to identify the types and number of each atom that makes up C7H8N4O2.
7 carbon atoms, 8 hydrogen atoms, 4 nitrogen atoms, and 2 oxygen atoms
Plants perform a chemical reaction known as photosynthesis in which light energy is converted into chemical energy in the form of sugars. The chemical equation for photosynthesis is displayed.
Which statements accurately describe the chemical reaction process that plants undergo?
A. No new substances are produced during the process of photosynthesis since it is a chemical reaction.
B. A new substance is produced during photosynthesis because photosynthesis is a chemical reaction.
B. A new substance is produced during photosynthesis because photosynthesis is a chemical reaction.
Photosynthesis is the process by which green plants use carbon dioxide and water to make nutrients. The chemical equation for photosynthesis is shown.
6CO2 + 6H2O + sunlight → C6H12O6 + 6O2
Which statement about the photosynthesis reaction is true?
A. There are 18 atoms of oxygen in the reaction.
B. There are more atoms in the products than in the reactants.
C. There are the same number of carbon and oxygen molecules.
A. There are 18 atoms of oxygen in the reaction.