What is the smallest unit of matter in chemistry
The atom
Which is physical: ice melting or iron rusting
Ice melting
What is the density of an object with a mass of 20 g and a volume of 5 mL?
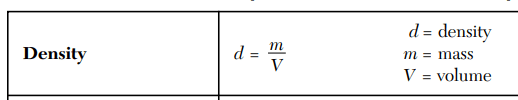
4 g/mL
How many sig figs in this measurement: 0.009100
4
A student claims that salt water is a homogeneous mixture. What simple evidence could they use to support this?
Salt water looks the same throughout
Salt water is an example of what kind of mixture?
Homogenous mixture
Define chemical property and give one example
Something that describes matter that can only be determined by a chemical rxn (reactivity, flammability, toxicity)
A student measured the density of aluminum as 2.70 g/mL. The accepted value is 2.71 g/mL. Calculate the percent error.
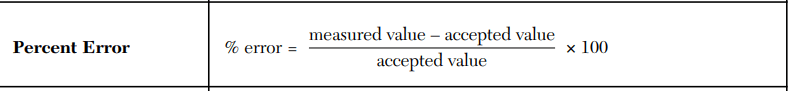
-0.37%
Convert to standard notation: 5.6 x 105
560,000
Write a claim about whether tearing paper is a physical or chemical change.
Physical change
Classify the following as elements or compounds
-Oxygen
-Gold
-Water
O- Element
Au- Element
H2O- Compound
Which property is intensive?
a) Mass
b) Length
c) Density
d) Height
c) Density
A cube of metal has a volume of 8 cm3 and a mass of 63.5 g.
Determine the density and identify if the cube is copper (8.96 g/mL), aluminum (2.70 g/mL), or iron (7.87 g/mL).
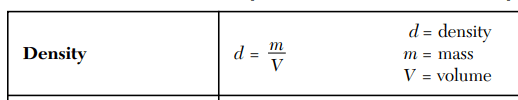
Iron (7.94)
Convert 4.3 L to mL
4,300 mL
In science, what is a “claim”?
The answer to your question
Heterogenous mixtures can be separated by physical means, give three examples of separation methods
filtration, magnetism, evaporation, chromatography, distallation
Identify whether each is a physical or chemical property: (a) Density, (b) Reactivity with acid, (c) Melting point.
a- Physical
b- Chemical
c- Physical
A student calculates a density of 7.2 g/mL, but the accepted density is 8.0 g/mL. Calculate the percent error and comment on whether the measurement is accurate or not.
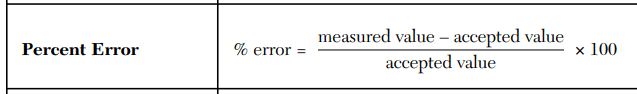
-10%
Convert to sci notation: 0.000891
8.91 x 10-4
A student claims that dissolving sugar in water is a chemical change. Provide one piece of evidence and reasoning to support or refute this claim.
Must have both!
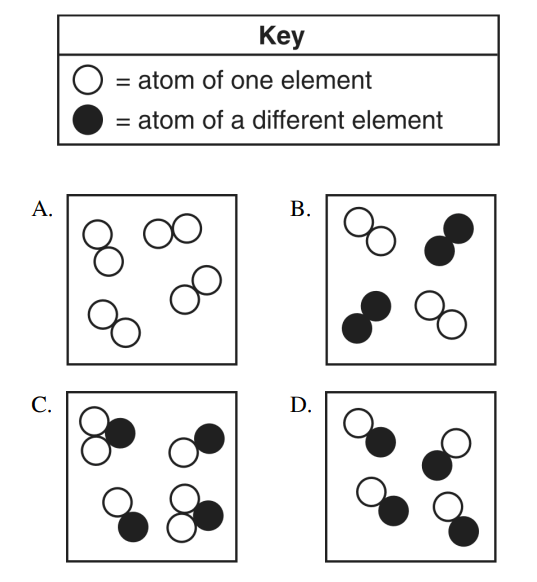
Classify each as an element, compound, or mixture
A: Element
B: Mixture of elements
C: Mixture of compounds
D: Compound
Explain why burning wood is a chemical change, not a physical change.
Cannot get the original back
A metal cylinder has a mass of 63.5 g and displaces 22.7 mL of water.
Identify the metal given: Cu = 8.96 g/mL, Al = 2.70 g/mL, Fe = 7.87 g/mL.
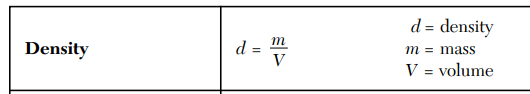
Aluminum
Convert 198,000 nanometers to meters
0.000198 m
A student claims their percent error shows high accuracy. The accepted density is 8.96 g/mL; their result was 8.90 g/mL. Calculate % error to support this claim.
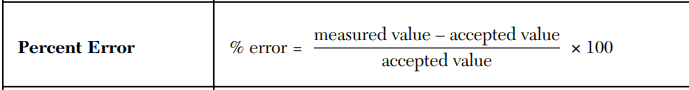
-0.67% which is small.