A measurement of how compact matter is in a given volume. Determines whether an object will sink or float in water.
What is Density?
Which substance is the most Acidic? Which is most Basic?
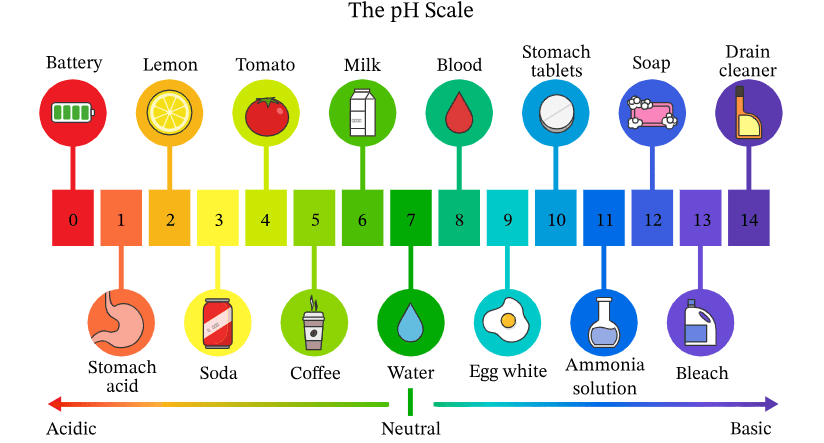
Battery Acid - most acidic (pH = 0)
Drain Cleaner - most basic (pH = 14)
Label each image with the state of matter.
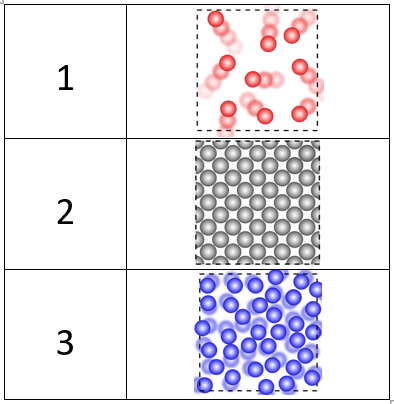
1 - Gas
2 - Solid
3 - Liquid
Anything that has mass and volume.
What is Matter?
How many moons does Saturn have?
145
The Volume of just ONE red ball.
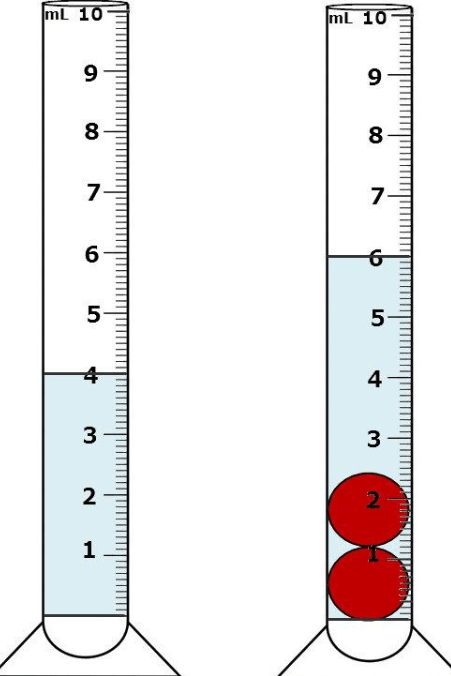
What is 1 mL?
How easily one substance will chemically combine or react with another substance. (hint: it's a chemical property)
Ex: Iron and Oxygen
What is Reactivity?
For each statement below, determine if it describes a solid, liquid or gas.
1. No definite shape but definite volume (medium amount of energy)
2. Definite shape AND volume (low energy)
3. No definite shape or volume (high energy)
1. Liquid
2. Solid
3. Gas
Can be observed with 5 senses or by taking measurements, WITHOUT changing the substance.
What are Physical Properties?
In what year did the Wright brothers achieve the first powered flight?
1903
A student has a block of wood with a volume 11 mL and a mass of 44 grams. Calculate the density of the block of wood.
4 g/mL
How easily heat or electricity can flow through a substance. (hint: its a Physical Property)
Ex: Metals are very good at this.
What is Conductivity?
Label each statement with the correct state of matter.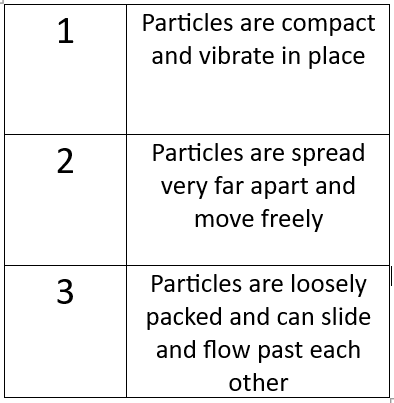
1 - Solids
2 - Gases
3 - Liquids
Can ONLY be observed by trying to change the substance into something brand new.
What are Chemical Properties?
In what year was the first iPhone released?
2007
A student has a cube of metal with a mass of 100 grams and a volume of 5 cm3. What is the density of the substance?
20 g/cm3
Which of the following are Physical Properties of matter.
- Melting Point
- Reaction with Water
- Combustibility
- Density
Melting Point, Density
As temperature _______________, particles speed up and spread out.
Increases
Identify the Phase Change term described in each statement below.
1. Solid to Liquid
2. Liquid to Gas
3. Gas to Liquid
1. Melting
2. Evaporation / Boiling
3. Condensation
How many different time zones are there in Russia?
(continental USA has 4)
11
Four substances are poured into a Graduated Cylinder. Based on their Densities, in what order would they separate from top (float) to bottom (sink)?
Substance A - 1.0 g/mL
Substance B - 1.4 g/mL
Substance C - 1.2 g/mL
Substance D - 0.9 g/mL
1. Substance D (floats at the top)
2. Substance A
3. Substance C
2. Substance B (sinks to the bottom)
Which of the following are Chemical Properties of matter?
- Acidity / Basicity
- Boiling Point
- Flammability
- Luster (how shiny)
Acidity / Basicity
Flammability
Label each statement with the correct state of matter.
1. Particles have LOTS of energy and motion
2. Particles have MEDIUM energy and motion
3. Particles have LITTLE energy and motion
1. Gas
2. Liquid
3. Solid
How easily something can catch on fire.
A property of wood, charcoal, and gas.
What is Combustibility?
How many years did the construction of the Great Wall of China span?
About 2,000 years
Gold has a known density of 20 g/mL. If a single nugget has a volume of 3 mL, what is it's mass?
60 grams
Which two samples are made of the same substance?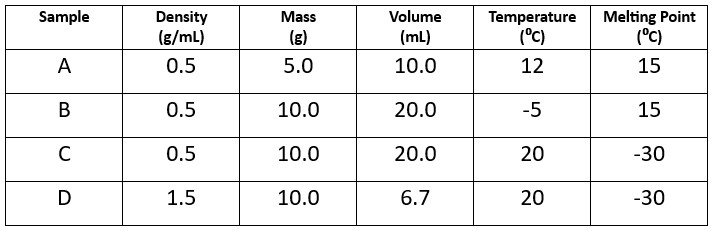
Sample A and B
The Kinetic Molecular Theory (KMT) describes how particles in matter behave. List TWO statements of the KMT.
1. All matter is made of particles
2. Particles are in constant and random motion
3. Particles collide with each other and the walls of their container
Explains how particles inside matter behaves.
Kinetic Molecular Theory (KMT)
What is the total number of chapters in the Harry Potter book series?
199