Define accuracy
How close our measurements are to the accepted/actual value
What is an intensive property?
Property that doesn't change with the amount of substance; is unique to the substance
What is density?
How compact the atoms are in a substance
I think that the mystery orange liquid left on my desk is orange juice. Is that an inference or an observation?
Inference
How do we determine how many decimal places to report a measurement to?
We leave one uncertain digit
Define precision
How close our measurements are to each other
What is an extensive property?
Property that DOES change with the amount of substance
I have a rock with a mass of 17.3 g and a volume of 11.8 cm3. What is the density of the rock?
1.47 g/cm3
Make three qualitative observations about this classroom
# ppl/desks/chairs in the classroom
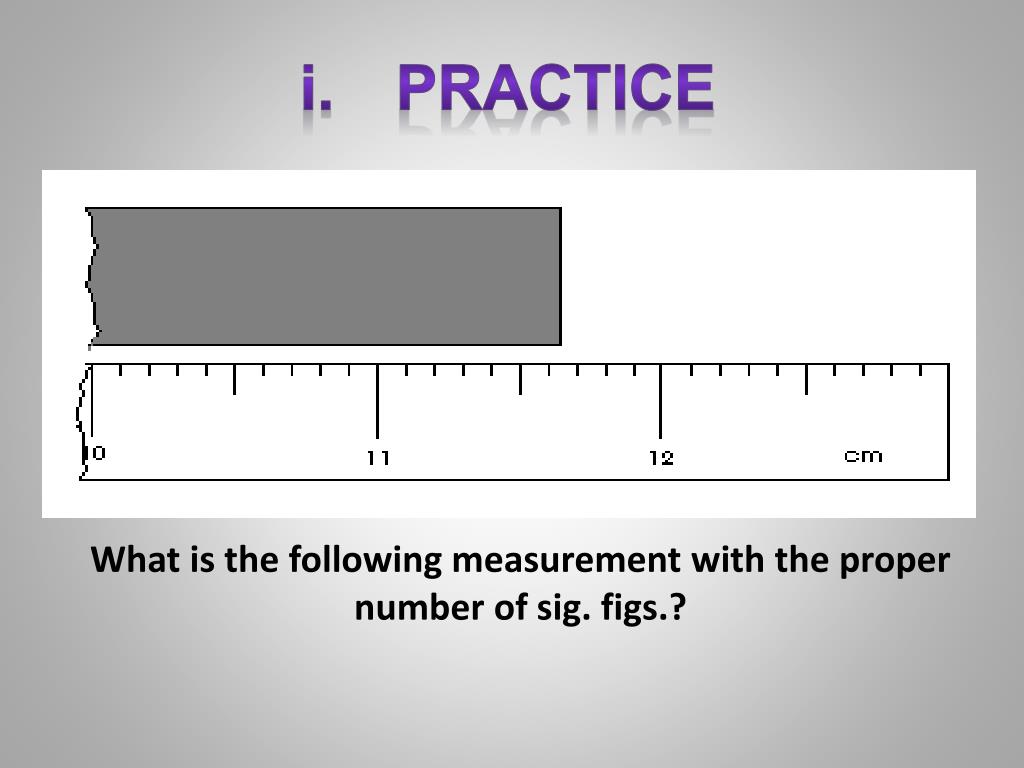
11.6x cm
Ms. Gelfand calculates the density of water, and gets 0.97 g/mL, 0.98 g/mL, and 1.03 g/mL. Are these measurements accurate, precise, both, or neither?
Accurate but not precise
Give three examples of intensive properties
odor, taste, density, boiling/melting/freezing point, texture, etc.
I have a solid with a density of 0.94 g/cm3. Will it sink or float in water? Why?
Float; density is less than that of water (1.0 g/mL)
Make three qualitative observations about this classroom
Color of the walls, bright/dim, etc.
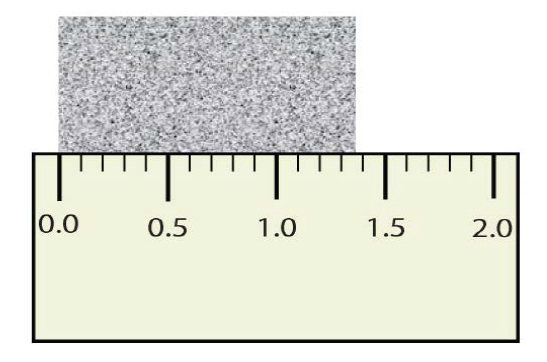
Report the measurement in the beaker with the correct number of sig figs

2.6x mL
2.69%
Give three examples of an extensive property
mass, weight, volume
I want to find the density of an irregularly shaped solid. I know its mass is 50g, but I'm having trouble measuring its length, width, and height to find the volume. How can I find the volume of the solid?
Water displacement
Which of the following is not an observation:
a. The object is red
b. The object is 12 cm long
c. The object is a marker
d. The object is cylindrical
Give this measurement to the correct number of significant figures. This measurement is given in inches.
1.3x in
Which piece of glassware yields the most precise measurements? Give TWO reasons.
Graduated cylinder
- More tick marks
- Lowest percent error in the measurement lab
Ms. Gelfand has a blue liquid in a beaker that she forgot to label. Think of two experiments she could do to try and figure out the identity of the unknown blue liquid.
Any experiment looking at an intensive property
I have a solid with the following dimensions:
Mass: 58 g
Length: 8 cm
Width: 4 cm
Height: 5 cm
What is the density of the solid?
0.3625 g/cm3
In a CER, should each of the following be composed of an inference, observation, or both?
Claim:
Evidence:
Reasoning:
Claim: Inference
Evidence: Observation
Reasoning: Both
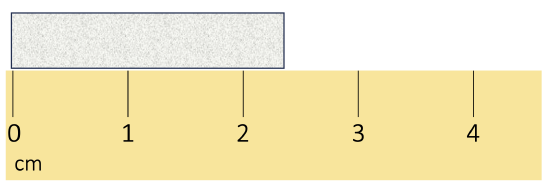
Report this measurement to the correct number of sig figs.