What is a homogenous mixture?
Composition is uniform. (You cannot see the substances in the mixture/it is all dissolved together)
Where are unsaturated solutions found on a solubility chart?
UNDER the line
What is a physical change?
A change in an object or substance's physical appearance but not its chemical make up. (It can be reversed!)
What is mass?
The amount of mass in an object
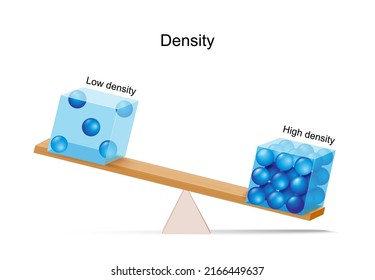
T/F: An object with a low density has a high mass.
FALSE:
High mass = high density.
What it is a heterogenous mixture?
Composition is NOT uniform (you can see the substances that make up the mixture/you can separate it easily)
Where are saturated solutions on the solubility chart?
ON the line
What is a chemical change?
The reaction causes a new substance to form. (It CANNOT be reversed)
What is volume?
The amount of space an object or substance takes up.
Order from LEAST DENSE to MOST DENSE
D
C
A
B
mixture or pure substance?

Pure substance: Compound Element
Where are supersaturated solutions found on the solubility chart?
ABOVE the line
Chemical or physical change?
Baking a cake
Chemical change
Which one has more mass?
A cup of water OR a bottle of flavored water
Flavored water
Which object is the most dense?
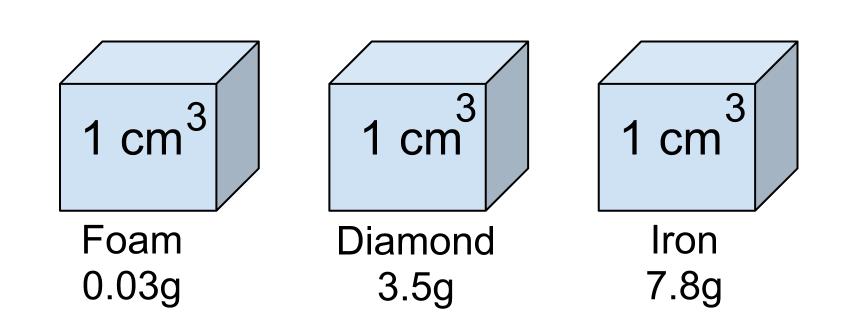
Iron
Can salt water be separated?
Yes, by evaporation
A solution of 30g of KClO3 in 100g of water at 40°C is....
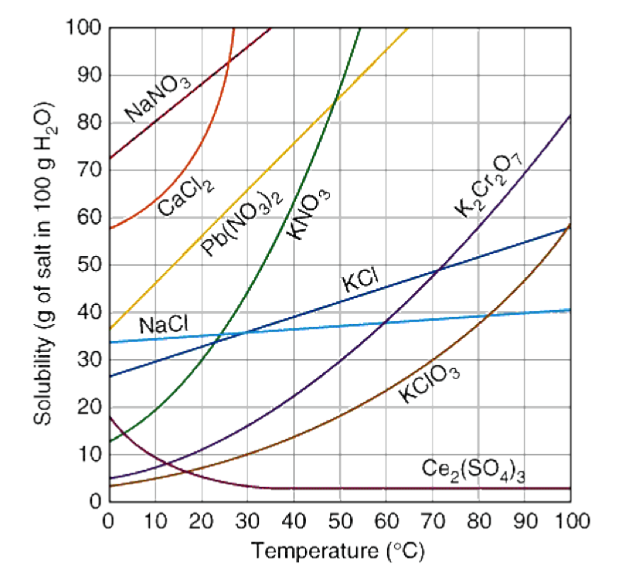
Supersaturated
Chemical or physical change?
Turning water into water vapor
What is the volume of a cube with a side length of 4cm?
64cm3
The amount of mass in a given space.
How can you separate oil that is floating on top of water?
Decantation
A solution with 70g of K2Cr2O7 in 100g of water heated at 80°C is...

Saturated
Chemical change or physical change?
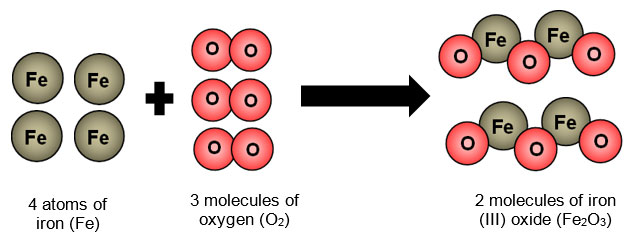
Chemical change
A rock is placed inside of a graduated cylinder containing water. After the rock is placed inside, the water level increases by 10mL. What physical property can be measured by this increase in water level?
Volume
The 3 cubes below are entirely made up of iron. Which one is more dense?
Small Medium Large
Large
It has a larger volume that is filled with more matter (iron).