The amount of matter that something is made of
What is mass?
How many mL is 1.8 L?
1800 mL
A property of matter that can be observed or measured without changing the identity of the matter
What are physical properties?
the force that the gas exerts on the walls of its container
What is pressure?
Solid to gas (i.e. dry ice)
What is sublimation?
Combination of substances that are not chemically combined
What is a mixture?
The study of matter and energy
What is Physical Science?
A force of attraction between objects that is due to their masses
What is gravity?
How many grams is 832 mg?
0.832 g
A property of matter that describes a substance's ability to change into a new substance with different properties
What are chemical properties?
A liquid's resistance to flowing (like honey)
What is viscosity?
Reaction that release heat
What is exothermic?
A group of elements that are shiny, conduct electricity and heat, are ductile and malleable.
What are metals?
A series of steps that scientists use to answer questions and solve problems.
What is the scientific method?
The tendency of an object to resist any change in motion
What is inertia?
What is the concentration of a solution that has 27 g of NaCl dissolved in 500 mL of water?
0.054 g/mL
Motion of the particles in this phase slide and roll past each other
What is a liquid?
Law that says Volume increases as pressure decreases at a constant temperature.
What is Boyles Law?
how does pressure affect boiling point?
the lower the pressure, the lower the temperature at which water will boil
pure substance that cannot be separated into simpler substances by physical or chemical means
What is a compound?
amount of solute needed to make a saturated solution using a given amount of solvent at a certain temp
What is solubilitiy?
A measure of the gravitational force exerted on an object; usually by the Earth
What is weight?
64.5 g/cm^3
A solid that is made up of crystals in which particles are arranged in a regular, repeating pattern like ICE, SALT, SUGAR
What is a crystalline solid?
the law that states that for a fixed amount of gas at a constant pressure, the volume of the gas increases as the temperature of the gas increases
What is Charles Law?
boiling vs vaporization
vaporization- evaporation on the surface
boiling evaporation throughout the whole liQuid
Compounds can only be broken down by ______ changes
What is chemical?
99% of matter in universe
no definite shape or volume
particles move with so much speed and force that they collide and break apart
What is plasma?
How does the mass of two objects and the distance between them affect gravity?
greater the mass and the shorter the distance, the stronger gravitational force to come together
Can 50 g of sodium chloride dissolve in 100 mL of water at 60°C?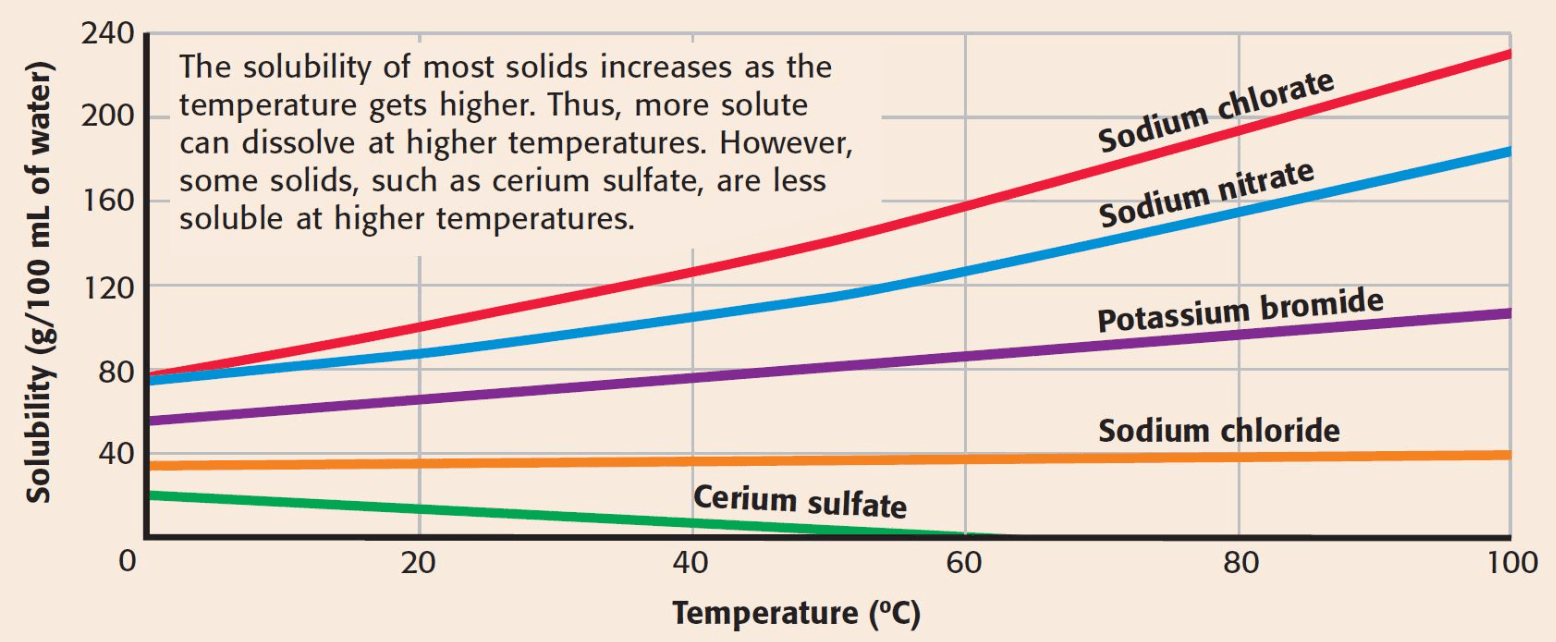
No
A solid made up of particles that are not arranged in a regular patter like RUBBER, WAX, GLASS
What is an amorphous solid?
Amount of matter in a given space (or grams per volume).
What is density?
what happens to temperature during a phase change?
Stays the same
mixture that appears to be a single substance, but is composed of particles of two or more substances that are distributed evenly amongst each other
What is a solution?
the curve at a liquid's surface by which one measures the volume of the liquid
What is a meniscus?
Solubility of most solids ______ as temperature increases. Whereas, solubility of gases _____ as temperature increases.
What is increases/ decrease?